Standard Reduction Potential
In chemistry, many reactions involve the transfer of electrons from one substance to another. These reactions are called redox reactions. Some substances readily give up electrons (oxidation), while others easily accept them (reduction). But how do we know which substance is more likely to gain or lose electrons?
This is where the standard reduction potential or standard electrode potential comes in. It measures how easily a substance gains electrons and gets reduced.
Why is Standard Reduction Potential Important
Standard reduction potential helps us:
- Predict whether a redox reaction will occur spontaneously.
- Determine the direction of electron flow in an electrochemical reaction.
- Calculate the overall energy produced in a redox process.
Predicting Spontaneity Using Standard Reduction Potential
We compare the standard reduction potentials (E°) of different substances to predict whether a reaction occurs spontaneously.
- The more positive the E° value, the more readily a substance gains electrons (undergoes reduction).
- The more negative the E° value, the more likely a substance loses electrons (undergoes oxidation).
Scientists use the standard hydrogen electrode (SHE) as a reference. Its standard reduction potential is 0.00 V, and all other substances are measured relative to this value.
Standard Reduction Potential Table
| Half-Reaction | E° (V) at 25°C |
|---|---|
| F₂ + 2 e⁻ → 2 F⁻ | +2.87 |
| MnO₄⁻ + 8 H⁺ + 5 e⁻ → Mn²⁺ + 4 H₂O | +1.51 |
| Cl₂ + 2 e⁻ → 2 Cl⁻ | +1.36 |
| O₂ + 4 H⁺ + 4 e⁻ → 2 H₂O | +1.23 |
| Br₂ + 2 e⁻ → 2 Br⁻ | +1.07 |
| NO₃⁻ + 4 H⁺ + 3 e⁻ → NO + 2 H₂O | +0.96 |
| Ag⁺ + e⁻ → Ag | +0.80 |
| Fe³⁺ + e⁻ → Fe²⁺ | +0.77 |
| O₂ + 2 H₂O + 4 e⁻ → 4 OH⁻ | +0.40 |
| Cu²⁺ + 2 e⁻ → Cu | +0.34 |
| Cu⁺ + e⁻ → Cu | +0.52 |
| I₂ + 2 e⁻ → 2 I⁻ | +0.54 |
| 2 H⁺ + 2 e⁻ → H₂ (SHE) | 0.00 |
| Pb²⁺ + 2 e⁻ → Pb | -0.13 |
| Sn²⁺ + 2 e⁻ → Sn | -0.14 |
| Ni²⁺ + 2 e⁻ → Ni | -0.25 |
| Co²⁺ + 2 e⁻ → Co | -0.28 |
| Fe²⁺ + 2 e⁻ → Fe | -0.44 |
| Cd²⁺ + 2 e⁻ → Cd | -0.40 |
| Cr³⁺ + 3 e⁻ → Cr | -0.74 |
| Zn²⁺ + 2 e⁻ → Zn | -0.76 |
| Mn²⁺ + 2 e⁻ → Mn | -1.18 |
| Al³⁺ + 3 e⁻ → Al | -1.66 |
| Mg²⁺ + 2 e⁻ → Mg | -2.37 |
| Na⁺ + e⁻ → Na | -2.71 |
| Ca²⁺ + 2 e⁻ → Ca | -2.87 |
| K⁺ + e⁻ → K | -2.92 |
| Li⁺ + e⁻ → Li | -3.04 |
Calculating the Standard Cell Potential
The standard cell potential (E°cell), measured under standard conditions (1 atm for gases and 1 M for solutes), typically at 25 °C, is determined by subtracting the standard reduction potential of the anode half-reaction (E°anode) from that of the cathode half-reaction (E°cathode).
E°cell = E°cathode − E°anode
- If E°cell is positive, the reaction is spontaneous (it can generate energy).
- If E°cell is negative, the reaction is non-spontaneous (it requires external energy).
This principle is fundamental in batteries.
Example Calculations
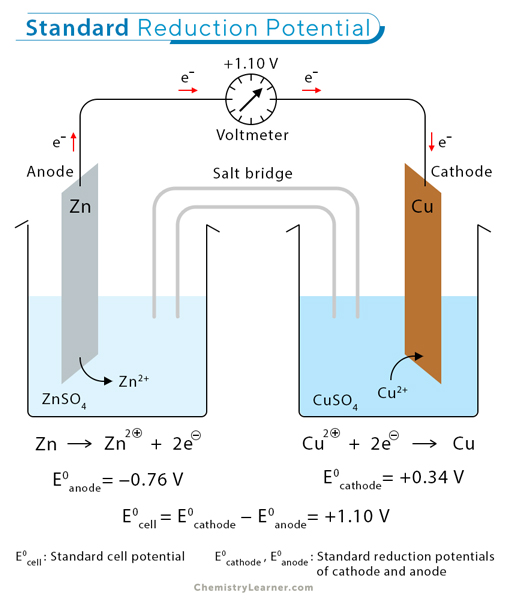
Example 1: Zn/Cu Electrochemical Cell
Step 1: Identify the Half-Reactions
Reduction (Cathode): Cu2+ + 2 e− → Cu
Oxidation (Anode): Zn → Zn2+ + 2 e−
Step 2: Find Standard Reduction Potentials from the Table
E°Cu2+/Cu = +0.34 V
E°Zn2+/Zn = −0.76 V
Step 3: Use the Formula to Calculate E°cell
E°cell = +0.34 V − (−0.76 V) = +1.10 V
The reaction is spontaneous.
Example 2: Ag/Zn Electrochemical Cell
Step 1: Identify the Half-Reactions
Reduction (Cathode): Ag⁺ + e⁻ → Ag
Oxidation (Anode): Zn → Zn2+ + 2 e−
Step 2: Find Standard Reduction Potentials from the Table
E°Ag+/Ag = +0.80 V
E°Zn2+/Zn = −0.76 V
Step 3: Use the Formula to Calculate E°cell
E°cell = +0.80 V − (−0.76 V) = +1.56 V
The reaction is spontaneous.
Example 3: Cu/Fe Electrochemical Cell
Step 1: Identify the Half-Reactions
Reduction (Cathode): Cu2+ + 2 e− → Cu
Oxidation (Anode): Fe²⁺ → Fe³⁺ + e⁻
Step 2: Find Standard Reduction Potentials from the Table
E°Cu2+/Cu = +0.34 V
E°Fe3+/Fe2+ = +0.77 V
Step 3: Use the Formula
E°cell = +0.34 V − (+0.77 V) = -0.43 V
The reaction is not spontaneous.
Example 4: Mg/Ag Electrochemical Cell
Step 1: Identify the Half-Reactions
Reduction (Cathode): Ag+ + e− → Ag
Oxidation (Anode): Mg → Mg2+ + 2 e−
Step 2: Find Standard Reduction Potentials from the Table
E°Ag+/Ag = +0.80 V
E°Mg2+/Mg = -2.37 V
Step 3: Use the Formula to Calculate E°cell
E°cell = +0.80 V − (−2.37 V) = +3.17 V
The reaction is spontaneous.
Applications
- Batteries – Determines the voltage of different battery types (alkaline, lithium-ion).
- Corrosion & Prevention – Explains metal rusting and protective coatings (e.g., galvanization).
- Electroplating – Used for metal coating (e.g., gold and silver plating).
- Fuel Cells – Helps design hydrogen fuel cells for electric vehicles.
- Water Purification – Used in electrochemical methods to remove contaminants.
- Metal Extraction – Assists in extracting metals like aluminum and copper from ores.