Solvation
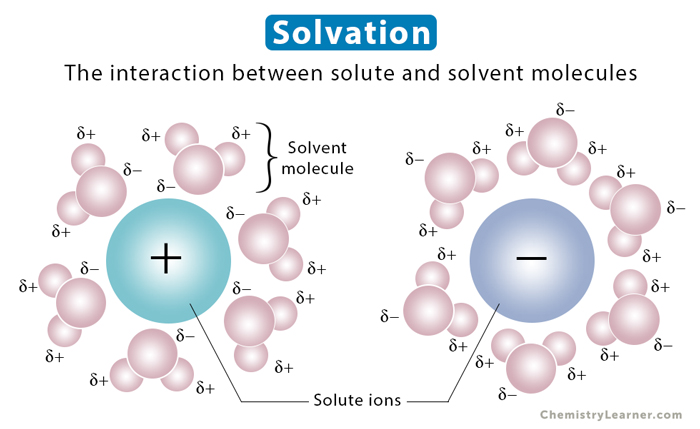
Solvation refers to the process of the interaction between a solute and a solvent. The solvent’s polarity is critical in determining how well it solvates the solute. The solute particles interact with the solvent. The nature of this interaction influences many properties, such as solubility and reactivity. Besides, it also affects the viscosity and density of the solvent. When the solvent is water, the process is known as hydration.
Process
Solvation occurs when the intermolecular forces between the solvent and solute particles are more significant than the intramolecular forces holding the solute particles together. Therefore, the greater the intermolecular forces, the stronger the attraction. The solvent particles separate the solute particles, pull them apart, and surround them. The surrounded solute particles then move away from the rest of the solute particles and get into the solution.
Both ions and neutral molecules participate in solvation. Ions exert strong forces on solvent molecules. A concentric shell of solvent surrounds them. The solvent and solute molecules reorganize into solvation complexes through bond formation, hydrogen bonding, and van der Waals forces. Polar molecules can easily solvate ions because they can position the partially charged portion of the molecule towards the ion due to electrostatic attraction.
Characteristics
- Polar solvents solvate polar solutes. Non-polar solvents cannot solvate them. For example, sodium chloride is solvated by water and not by benzene.
- Non-polar solvents solvate non-polar solutes. Polar solvents cannot solvate them. For example, icosane is solvated by benzene and not by water.
- Some molecules, like soap, have a polar and non-polar parts. The non-polar part attracts non-polar molecules such as grease. On the other hand, the polar part is solvated by water. This unique property of soap allows one to wash dirty dishes after a meal.
Example
The dissolution of sodium chloride (NaCl) into water is a simple example of solvation. In solid sodium chloride, the Na+ cation and Cl– anion are bonded through ionic bonding. The NaCl crystal lattice comprises Na+ ions surrounded by numerous Cl– and other Na+ ions. When NaCl dissolves in water, each ion attracts the polar water molecule and moves away from the lattice. When enough water molecules have been attracted, the ion has a solvent shell, and it dissolves. Therefore, NaCl is solvated.
Solvation Number
The solvation number is the number of solvent molecules bound to a solute species. For example, in hydrated ferrous chloride (FeCl2(H2O)4), the solvation number is 4.
Solvation Energy
Solvation energy is the energy associated with dissolving a solute in a solvent. It is positive if the dissolving process is endothermic and negative if it is exothermic. The solvation process will only be thermodynamically favored if the overall Gibbs free energy of the solution decreases, that is, less than the Gibbs free energy of the separated solvent and solute.