Sigma and Pi Bonds
The hybridization model can explain covalent bond formation in a molecule. Covalent bonds are formed by overlapping atomic orbitals, resulting in sigma and pi bonds. The two bonds differ in the way in which overlapping occurs. Various bond properties like bond length, bond energy, and bond enthalpy depend on how orbitals overlap.
Sigma Bond
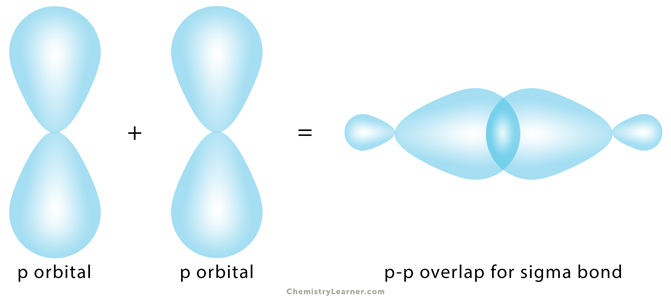
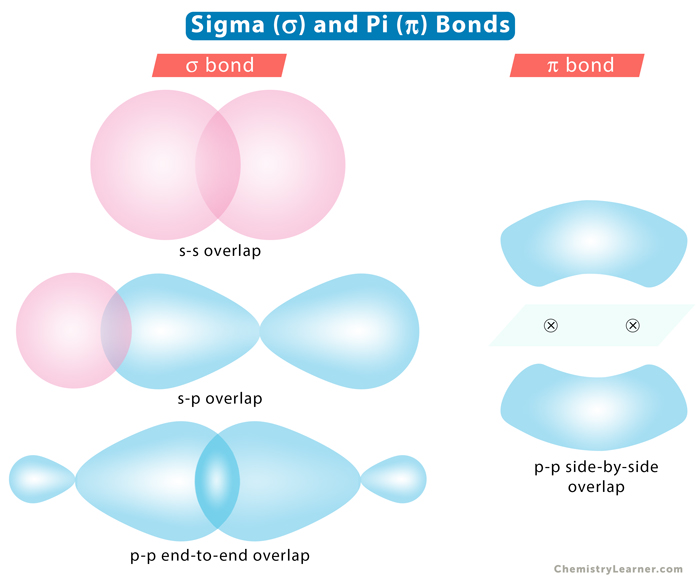
Sigma bond, represented by the Greek symbol σ, is formed by head-to-head overlapping of atomic orbitals along the internuclear axis. The electron density is concentrated between the nuclei of the bonding atoms. Sigma bond is the strongest covalent bond, owing to the direct overlapping of the contributing orbitals. The bonding electrons are usually referred to as sigma electrons.
Generally, all single bonds are sigma bonds. They can be formed via the following combinations of atomic orbitals.
1. s-s Overlapping
The s orbital from each atom participates in overlapping along the internuclear axis. The s orbitals must be half-filled before they overlap.
Example: In hydrogen (H2), the 1s orbital of each hydrogen atom overlap.
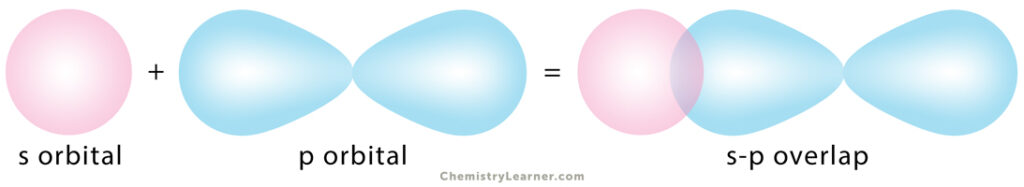
2. s-p Overlapping
One half-filled s orbital from one atom overlaps with one half-filled p orbital from another.
Example: In ammonia (NH3), the 2px, 2py, and 2pz orbitals of nitrogen (N) overlap with the 1s orbital of hydrogen (H).
3. p-p Overlapping
The half-filled p orbital from each atom participates in head-on overlapping along the internuclear axis.
Example: In chlorine (Cl2), the 2pz orbital of one chlorine atom overlaps with the 2pz orbital of another atom.
Lone pairs do not count as sigma bonds.
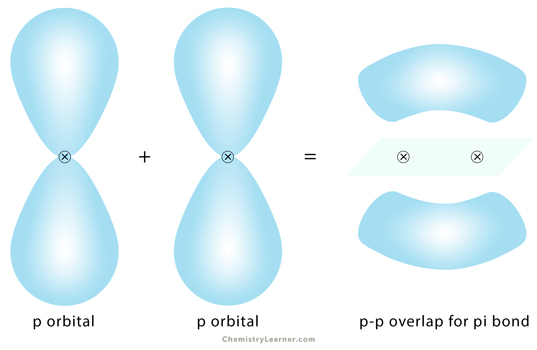
Pi Bond
Pi bond, represented by the Greek symbol π, is formed by side-by-side overlap, in parallel fashion, of the atomic orbitals. The electron density is concentrated above and below the plane of the nuclei of the bonding atoms. During the pi bond formation, the axes of the atomic orbitals are parallel to each other and perpendicular to the internuclear axis, that is, the plane of the two nuclei.
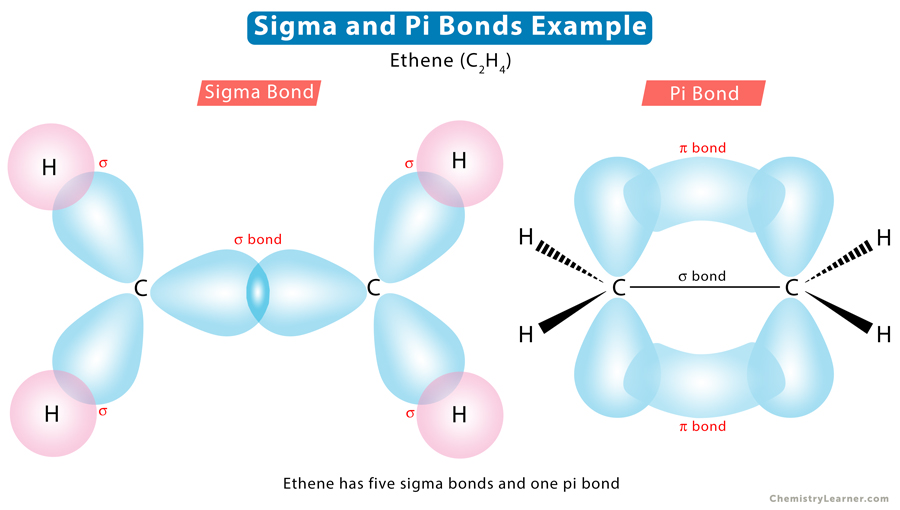
A double bond consists of one sigma and one pi bond. A triple bond is made up of one sigma and two pi bonds.
Example: In carbon dioxide (CO2), the 2px orbital of carbon will form a pi bond with the unhybridized 2px orbital of oxygen. Its 2pz orbital will form a pi bond with the other oxygen’s 2pz orbital. Therefore, CO2 has two sigma and two pi bonds.
How to Count Sigma and Pi Bonds?
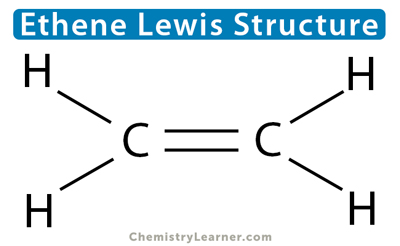
We must first know the Lewis structure to count the number of sigma and pi bonds in a molecule. Lewis dot structure is a simple way of representing a molecule’s outermost shell of electrons. In this structure, lines indicate chemical bonds. A single line represents a sigma bond, a double line represents one sigma bond and one pi bond, and a triple line represents one sigma bond and two pi bonds.
Let us take the example of ethene (C2H4) to understand how many sigma and pi bonds are present. According to its Lewis structure, carbon forms a double bond with another carbon atom and a single bond with the four hydrogen atoms. Since a double bond consists of one sigma and one pi bond, there are five sigma bonds and one pi bond in C2H4.
The following table illustrates the number of sigma and pi bonds in three selected hydrocarbons.
| Name of the compound | Molecular formula | Bond between carbon atoms | Total number of sigma and pi bonds |
|---|---|---|---|
| Ethane | C2H6 | 1 sigma | 7 sigma |
| Ethene or ethylene | C2H4 | 1 sigma, 1 pi | 5 sigma, 1 pi |
| Ethyne or acetylene | C2H2 | 1 sigma, 2 pi | 3 sigma, 2 pi |
Sigma vs. Pi Bonds
Are Pi or Sigma Bonds Stronger
The strength of a bond depends on the extent to which the orbitals overlap. The more pronounced the overlapping, the stronger the bond. The overlapping occurs more in sigma bonds than in pi bonds. Hence, sigma bonds are stronger than pi bonds. It is why reactions in organic chemistry mainly involve breaking the pi bond rather than the sigma bonds.
The following table compares and contrasts the two.
| Sigma Bond | Pi Bond |
|---|---|
| Formed by the head-to-head overlap of atomic orbitals | Formed by the side-to-side overlap of atomic orbitals |
| Strong and have high bond energies. | Relatively weak |
| Can exist independently | It must exist along with a sigma bond |
| Found in single, double, and triple bonds | Found in double and triple bonds |
| The overlapping orbitals can be pure or hybrid | The overlapping orbitals must be unhybridized |
FAQs
Ans. There are 13 sigma and 5 pi bonds in acetylsalicylic acid, also known as aspirin.
Ans. Benzene consists of 6 carbon atoms in a ring-like structure. Each carbon atom is connected through a sigma bond. Aside, each carbon atom is connected to hydrogen atoms through sigma bonds. Therefore, there are 12 sigma bonds. Also, alternate carbon-carbon atoms form a double bond. Hence, there are 3 pi bonds.
Ans. There are 19 sigma bonds and 4 pi bonds in vanillin.