Rate Law
The rate law of a chemical reaction gives a relationship between the reaction rate and the concentration of its reactants [1-4].
Rate Law Equation
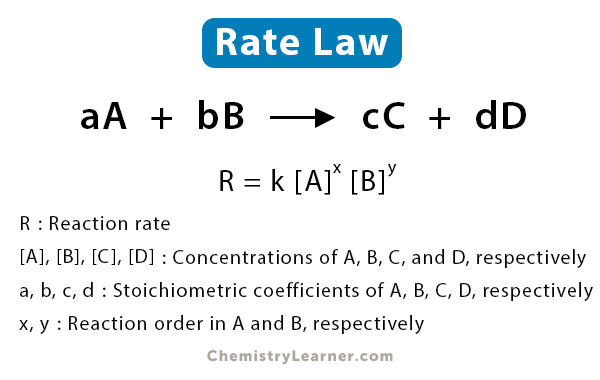
Consider the reaction between reactants A and B to form products C and D [1-5].
aA + bB → cC + dD
Where a, b, c, and d are the stoichiometric coefficients. The following expression gives the rate law.
R = k [A]x [B]y
Where [A] and [B] are the molar concentrations of A and B, respectively. k is the rate constant, which depends on the reaction type and temperature. The exponents x and y are the reaction orders in A and B, respectively, and the sum x + y gives the overall reaction order. The rate constant and the reaction order are determined experimentally, which means that the rate law can only be determined through experiments.
The extent to which changing the concentration affects the reaction rate depends on the reaction order. The following table summarizes the effect.
| Reaction order | Doubling the reactant’s concentration |
| 0 | will not affect the reaction |
| 1 | will double the rate |
| 2 | will quadruple the rate |
| 3 | will increase the rate by eight times |
Rate Law Examples [1-5]
1. Nitrogen dioxide (NO2) and carbon monoxide (CO) gases react to form nitric oxide (NO) and carbon dioxide (CO2).
NO2 (g) + CO (g) → NO (g) + CO2 (g)
The rate law for this reaction is given by
R = k [NO2]2
Therefore, the reaction is second-order in NO2 and zero-order in CO. The overall order is 2.
2. Hydrogen (H2) and nitric oxide (NO) gasses react to form nitrous oxide (N2O) and water (H2O).
H2 (g) + 2 NO (g) → N2O (g) + H2O (g)
The rate law for this reaction is
R = k [NO]2[H2]
Therefore, the reaction is second order in NO and first order in H2. The overall reaction order is three.
Units for Rate Constant
The units for rate constant k can be found through dimensional analysis. Generally, if the overall reaction order is n, the unit is Ln-1 mol1-n s-1. The following table gives the rate constant units [1-5].
| Overall reaction order (n) | Rate constant unit Ln-1 mol1-n s-1 |
|---|---|
| 0 | L-1 mol s-1 |
| 1 | s-1 |
| 2 | L mol-1 s-1 |
| 3 | L2 mol-2 s-1 |
The units of k can also be expressed in terms of molarity instead of moles per liter (mol L-1).
How to Determine Rate Law
To understand how to determine the rate law, let us take an example. Consider the following reaction [2]:
A + B → C
The initial rates and the concentrations of A and B are given in the table below.
| Test | Initial rate (mol L-1 s-1) | [A] (mol L-1) | [B] (mol L-1) |
|---|---|---|---|
| 1 | 2.73 x 10-5 | 0.1 | 0.1 |
| 2 | 5.47 x 10-5 | 0.2 | 0.1 |
| 3 | 2.71 x 10-5 | 0.1 | 0.2 |
Comparing tests 1 and 2, we find that the concentration of A is doubled and B is kept constant. Also, the rate constant does not change. Recall that the rate law is given by R = k [A]x [B]y.
Taking the ratios of the two rates:
R1/R2 = [A1/A2]x = (2.73 x 10-5)/(5.47 x 10-5) = (0.1/0.2)x
=> x = 1
Therefore, the reaction is first order in A.
Comparing tests 1 and 3, we find that B is doubled while A is kept constant. Taking the ratio of the two rates:
R1/R2 = [B1/B2]y = (2.73 x 10-5)/( 2.71 x 10-5) = (0.1/0.2)y
=> y = 0
Therefore, the reaction is zero-order in B.
The rate law is
R = k[A]
Now, let us determine the rate constant from any test.
k = R/[A] = 2.73 x 10-5 mol L-1 s-1 /0.1 mol L-1 = 2.73 x 10-4 mol L-1
Therefore, the rate law is
R = 2.73 x 10-4 mol L-1 [A]





Thanks and please what is the formular of rate law
It is mentioned in the article – R = k [A]x [B]y.