Radium
What is Radium
A highly radioactive and naturally-occurring metal, radium (pronounced as RAY-dee-em) is formed when uranium and thorium undergo disintegration in the environment. Represented by the chemical symbol Ra, it is classified in the family of alkaline earth metals [1]. It has 33 isotopes of which Ra-226 and Ra-228 have a half-life of 1600 years and 5.75 years, respectively [11].
Where is the Element Found
Since the element is found in most uranium ores, especially in Canada and Democratic Republic of Congo, it can be obtained as a byproduct of uranium refining. Another source of radium is the spent fuel rods of nuclear reactors [1].
History
Origin of its Name: It is derived from the Latin word ‘radius’ that means ray [1].
Who Discovered Radium: Renowned scientists Marie Curie and Pierre Curie were the discoverers [1]
When, Where, and How was it Discovered
Marie Curie had observed that pitchblende (uranium oxide) was more radioactive even after extracting uranium from it. In 1898, the Curie couple used some chemical separation techniques to isolate radium from ten tons of the pitchblende that displayed new lines in its atomic spectrum. They were fascinated to find it glow with a faint blue light in the dark due to its high radioactivity [1].
In 1911, Marie Curie and Andre Debierne carried out electrolysis of radium chloride where a mercury cathode was used to isolate the metal [1].
Identification |
|||
| Atomic number | 88 [1] | ||
| CAS number | 7440-14-4 [1] | ||
| Position in the periodic table [1] | Group | Period | Block |
| 2 | 7 | s | |
Classification, Properties and Characteristics of Radium
General Properties |
||
| Relative atomic mass | [226] [1] | |
| Atomic mass/weight | 226 atomic mass units [4] | |
| Molar mass | 223.018 g/mole [5] | |
| Mass Number | 226 | |
Physical Properties |
||
| Color/appearance | Silver white [1] | |
| Odor | Unknown [6] | |
| Melting point/freezing point | 696°C (1285°F) [1] | |
| Boiling point | 1500°C (2732°F) [1] | |
| Density | 5 g/cm3 [1] | |
| Normal phase at room temperature (solid/liquid/gas) | Solid [1] | |
Chemical Properties |
||
| Oxidation state/Oxidation number | +2[1] | |
Atomic Data of Radium (Element 88)
| Valence electrons | 2 [7] | ||||||
| Quantum numbers [7] | |||||||
| – n | 7 | ||||||
| – ℓ | 0 | ||||||
| – mℓ | 0 | ||||||
| – ms | -1/2 | ||||||
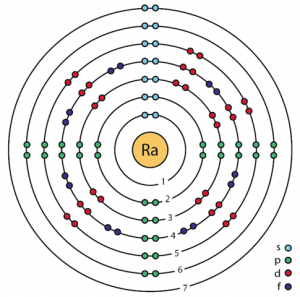
| Electron configuration (noble gas configuration) | [Rn] 7s2[1] | ||||||
| Atomic structure [4] | |||||||
| – Number of Electrons | 88 | ||||||
| – Number of Neutrons | 138 | ||||||
| – Number of Protons | 88 | ||||||
| Radius of atom | |||||||
| – Atomic Radius | 2.83 Å [1] | ||||||
| – Covalent Radius | 2.11 Å [1] | ||||||
| Ionic charge | +2 | ||||||
| Ionization energy [1]
(kJmol-1) |
1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| 509.29 | 979.051 | – | – | – | – | – | |
What is Radium Commonly Used for
- Ra implants are effective in treating oral cancer through internal radiation therapy. A mild radioactive form of the element, radium-223 is used in the treatment of prostate cancer that has spread to other parts of the body like bones [1, 8].
- A mixture of radium and beryllium is useful as a neutron source [1].
- The element is used to make night lamps, bulbs, and tube lights.
- Luminous paints containing radium has been used in dials of clocks, watches aircraft devices, and other instruments. However, the metal is now replaced with cobalt-60 as it poses hazardous effects due to emission of radioactive rays [1].
- Radium-226 undergoes decay by alpha emission into radon-222 that may have the potential to treat certain types of cancer, including lung cancer [2].
Does the Element Have Any Dangerous Effects
Long-term exposure to the highly radioactive metal can have toxic effects on health, resulting in cataract, anemia, brittle teeth, and cancer [9]. According to some studies, ingestion of Ra-224 and Ra-228 has been linked to bone sarcomas [3].
Interesting Facts
- The unit of radioactivity, Curie, is equal to the number of atoms in gram of radium-226 that undergoes decay in one second [2].
- As the research notes and documents of Ra have been exposed to the radioactive rays, they not safe even today to be handled for further studies [2].
- The activity of radium is about a million times more than uranium [2].
- In the early 1900’s, several women factory workers employed to paint watch dials and clock faces were contaminated with Ra, present in those luminous paints, due to regular exposure that resulted in adverse health effects followed by death [10].
Radium Cost
The price of pure Ra is somewhere between $100,000 and $120,000 per gram.
- References
- http://www.rsc.org/periodic-table/element/88/radium
- https://education.jlab.org/itselemental/ele088.html
- https://pubchem.ncbi.nlm.nih.gov/compound/radium#section=Other-Identifiers
- http://hobart.k12.in.us/ksms/PeriodicTable/radium.htm
- https://www.webqc.org/molecular-weight-of-Radium.html
- http://teacher.k12.de.us/~lettieri/pt/ra/ra.htm
- http://chemistry-reference.com/q_elements.asp?Symbol=Ra&language=en
- http://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/radiotherapy/internal/radioactive-liquid-treatment/radium-223
- https://www.atsdr.cdc.gov/phs/phs.asp?id=789&tid=154
- http://allthatsinteresting.com/radium-girls
- https://education.jlab.org/itselemental/iso088.html