Properties of Water
Water is a tasteless, odorless, and colorless liquid. It is widely abundant on Earth, with nearly ¾ of its surface covered with water. Water has several unique properties that make it special for all living organisms [1-4].
List of Properties of Water [1-4]
Structure
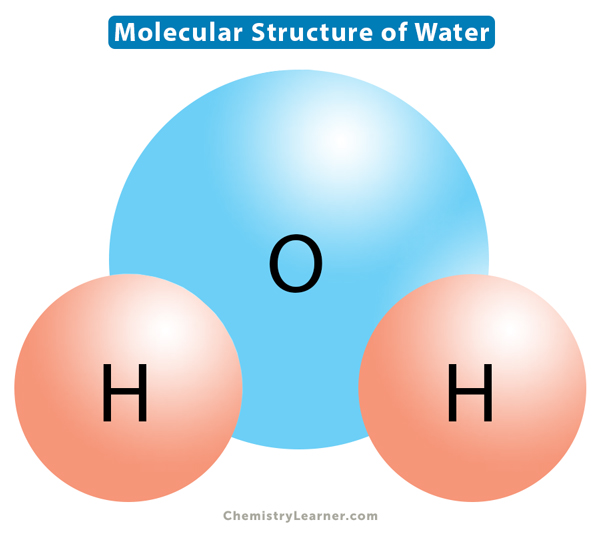
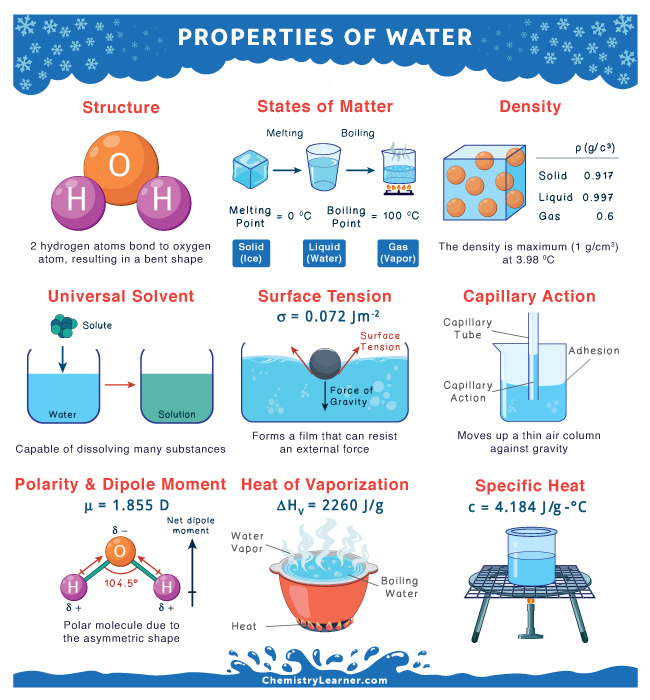
Water consists of two atoms of hydrogen and one atom of oxygen. Each of the two hydrogen atoms is covalently bonded with the oxygen atom, forming a tetrahedral shape. The water molecule is bent due to the repulsive effect of the lone pairs of electrons in oxygen. The H-O-H bond angle is 104.50.
Polarity and Dipole Moment
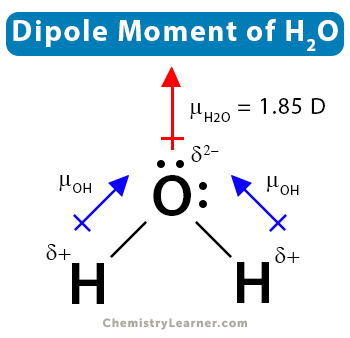
Water is a polar molecule because of its asymmetrical shape. The oxygen atom is more electronegative than the hydrogen atom. It attracts the bonding electrons towards itself and acquires a partially negative charge. On the other hand, the two hydrogen atoms acquire partially positive charges. Therefore, the O-H bond is polar. Water has a bent shape that gives rise to a net dipole moment of 1.855 D. Its molecules form hydrogen bonds because of its polarity.
Universal Solvent
Water is called a universal solvent. It dissolves many polar and ionic species. This property benefits living organisms since water carries essential nutrients to different functional parts. Although water dissolves many substances, it cannot dissolve oils, fats, and some hydroxides.
High Heat Capacity
Water has a high heat capacity, meaning it takes a lot of energy to heat water. Precisely, water absorbs 4184 Joules of heat per kilogram for its temperature to increase by 1 °C. This property helps moderate Earth’s temperature since water traps heat during the day and releases it gradually at night. As a result, Earth’s temperature does not vary highly.
High Heat of Vaporization
Water has a high heat of vaporization that helps us to cool down on a hot summer day. It requires 2260 Joules per gram to vaporize completely into steam. Sweat consists of water that evaporates from our skin. It carries excessive heat from our bodies, making us dry and comfortable. This process is known as evaporative cooling.
Cohesive Property and Surface Tension
Water molecules combine to form strong hydrogen bonds, resulting in cohesive forces. Each molecule is pulled from all directions. On the surface, the molecules are only pulled from the sides and the bottom, not from the top. Water forms a skin on the surface that can resist an external force. This property of water is known as surface tension. The value of surface tension is 0.072 J m2.
Adhesive Property and Capillary Action
Water molecules can also stick to surfaces. This property is known as adhesion. Water molecules adhering to the walls of a container cause an upward force at the edges and result in an upward meniscus. On the other hand, cohesion allows water molecules to stay together. Capillary action involves the movement of water molecules. It occurs when adhesion to the walls is stronger than the cohesion between the liquid molecules. It is essential for plants and trees; since water and all the dissolved nutrients can reach the top against gravity.
Density
Water compresses when it freezes. The density of ice is less than water, allowing ice to float on water. This phenomenon can be observed in lakes on a freezing day. A thick layer of ice covers the surface. The ice traps the heat from escaping the lake, preventing it from freezing. This way, fish and other living organisms can survive underwater. The following table shows the density values of the different states of water at 1 atm pressure.
| State | Density |
|---|---|
| Ice | 0.917 g/cm3 |
| Water | 0.997 g/cm3 |
| Vapor | 0.6 g/cm3 |
Solid State (Ice)
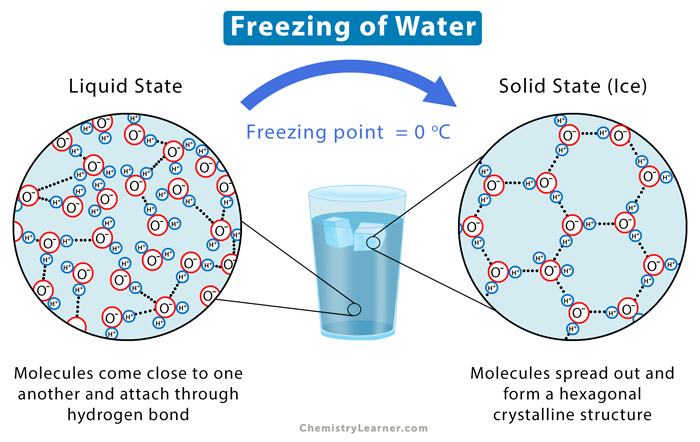
When water freezes, it forms ice. Ice is a dense substance where molecules are close to one another and form a hydrogen bond, resulting in a hexagonal crystalline structure. Pure ice is transparent. It can look opaque blue if soil particles and air bubbles are present. Ice is used for cooling, winter sports, and ice sculpting.
Liquid State
Water is in a liquid state at room temperature. It is very unusual for a compound not containing carbon to be liquid at room temperature. Water molecules form fewer hydrogen bonds than ice, allowing them to move quickly and form a dense structure.
Gaseous State (Water Vapor)
Water in the gaseous state forms hot steam. The molecules are separated as hydrogen bonds break up. The lack of hydrogen bonds makes steam very dangerous when it comes in contact with skin. Steam molecules carry the energy from hydrogen bond breaking. When they strike the face, the latter absorbs energy as the steam condenses into liquid water through an exothermic process, resulting in burns.
Freezing and Boiling Points
Water freezes at 0 oC and boils at 100 oC. The high boiling point is because water requires much energy to break the hydrogen bonds before it boils. Likewise, massive energy is required for ice to melt. Because ice and water require enormous energy to transform phase, melting and evaporation are slow. This property is vital for the ecosystem, especially living organisms, to survive underwater.
FAQs
Ans. Density is an important property of water because small changes in density result in a significant change in water pressure at great depths.
Ans. Density. Oil floats on water because it is less dense than water.
Ans. Polarity. Sugar can dissolve in water because sucrose is slightly polar and can form intermolecular bonds with the polar water molecules.
Ans. Cohesive property. Beads form because water molecules tend to attract each other and form bonds.






Very good
Ola, muito gratificante, especificamente, demonstravel, seguro….