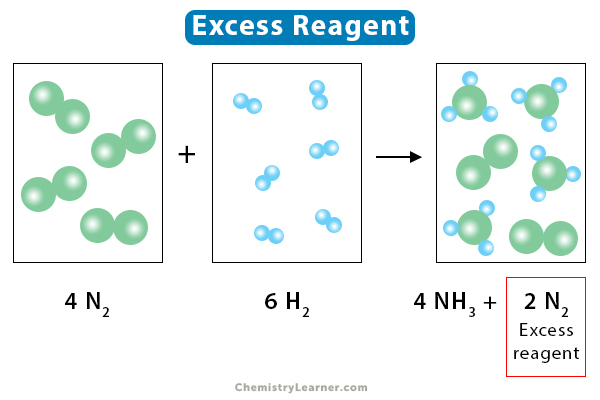
Potassium tert-butoxide
Potassium tert-butoxide is a chemical compound that is colorless in form with a strong base.
Potassium tert-butoxide Names
This compound is also known by other names like :
- Potassium t-butoxide
- Potassium tertiary butoxide
- Potassium tert-butylate
- Tert-butyl alcohol potassium derivative
Potassium tert-butoxide Chemical Formula
The chemical formula for this compound is C4H9KO.
Potassium tert-butoxide Molecular Weight
The molecular weight of this substance is 112.21.
Potassium tert-butoxide Density
The density of this compound is 0.902.
Commercial Availability and Laboratory Preparation
This chemical compound is available commercially both as a solid and as a solution. However, it is frequently made in laboratories because commercial samples are often sensitive and older packs of poor quality. It is manufactured by reacting dry tert-butanol with potassium metal. Solid potassium tert-butoxide is obtained by evaporation of these solutions followed by heating. One can purify the solid by sublimation at 220 °C and 1 mmHg.
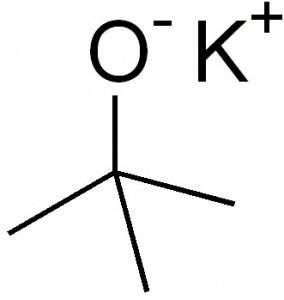
Picture 1 – Potassium tert-butoxide Image
Source – en.wikipedia.org
Potassium tert-butoxide Uses
Base Catalyst
Some reasons for the popularity of tert-butoxide as a base catalyst are:
- It is free from solvation
- This compound is stable for storage
- The structural deficiency of its hydrogen atoms for self-deprotonation
- Its relatively lesser tendency of undergoing SN2 reactions to develop into tert-butyl ethers
Potassium tert-butoxide can be used in manufacturing products like sidenafil and biodiesel. There is a large worldwide market for it as a low-cost strong base catalyst.
Organic Synthesis
It is useful in organic synthesis. The tert-butoxide group of compounds is found useful as a powerful, non-nucleophilic base. It is not as powerful as amide bases like lithium diisopropylamide, but stronger than potassium hydroxide. The steric bulk of the group resists it from taking part in nucleophilic addition, such as in a Williamson ether synthesis or an SN2 reaction.
Alkene Manufacture
The reaction of an alkylhalide with a strong base can be deployed for the manufacture of an alkene, provided that a mixture of isomers is not generated in the process. Potassium tert-butoxide is particularly suitable with a smaller amount of hindered substrates to avoid competing substitution.
Deprotonating and Dehydrohalogenation Reactions
Substrates such as terminal acetylenes and active methylene compounds can be deprotonated by it. It is also useful in Dehydrohalogenation reactions.
Anionic Activation of C-H, O-H and N-H Bonds
Solutions of Potassium tert-butoxide are especially appropriate for the anionic activation of C-H, O-H and N-H bonds, causing reactions such as Stereoisomerisation, Olefin Isomerisation and H/D/T exchange.
Material Safety Data Sheet (MSDS)
This is a stable compound; however, it reacts strongly with water and acids and can lead to fire. It is not compatible with substances like
- Water
- Acids
- Alcohols
- Strong oxidizing agents
- Carbon dioxide
- Ketones
It is injurious if ingested or inhaled. It can cause acute burns and severe damage to tissues of mucous membranes, respiratory tract, skin and eyes. Those using this compound are advised to wear safety glasses and gloves and work in a well-ventilated environment.
References
- References
- http://chemicalland21.com/industrialchem/organic/potassium-tert-butoxide.htm
- http://pubs.acs.org/doi/abs/10.1021/cr60287a004
- http://www.sciencedirect.com/science/article/pii/S0040402010014705
- http://www.alkalimetals.com/msds/POTASSIUM%20tert-BUTOXIDE_MSDS.pdf
- http://www.flintbox.com/public/project/6988/