Pinacol Rearrangement
Definition: What is Pinacol Rearrangement?
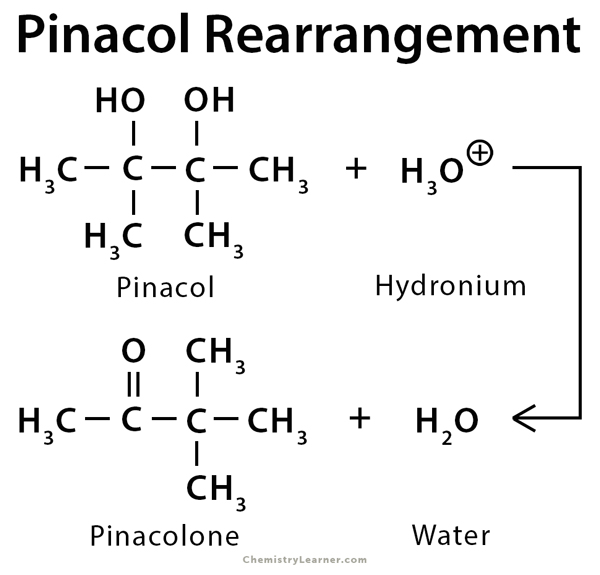
Pinacol is a compound which has two hydroxyl groups, each attached to a vicinal carbon atom. Its IUPAC name is 2,3-dimethyl-2,3-butanediol. Pinacol rearrangement is a 1,2-rearrangement of pinacol via carbon group migration to produce a ketone, often with acid catalysis. The resulting ketone is known as pinacolone or 3,3-dimethyl-2-butanone. Pinacol rearrangement is regioselective. The major or only product is derived from the rearrangement of the more stable carbocation [1-3].
The history of this reaction goes back to 1860 when German chemist Wilhelm Rudolph Fittig first discovered it.
Mechanism of Pinacol Rearrangement
This reaction occurs with a variety of fully substituted 1,2-diols. It involves the formation of a carbanion ion intermediate that subsequently undergoes a rearrangement [4-7].
Applications of Pinacol Rearrangement
Pinacol rearrangement is used to produce pinacolone, which is an essential ketone in organic chemistry. It is a precursor to triazolylpinacolone in the synthesis of pesticide, fungicide, and herbicide.
References
- Definition – Chem.libretexts.org
- Definition – Chem.ucla.edu
- Definition – Chemistry.msu.edu
- Mechanism – Organic-chemistry.org
- Mechanism – Chem.libretexts.org
- Mechanism – Chemtube3d.com
- Mechanism – Researchgate.net