Ozonolysis
Definition: What is Ozonolysis?
Ozonolysis is an organic reaction where the pi bonds of unsaturated olefins like alkenes and alkynes are cleaved with ozone (O3). It is a type of cycloaddition that destroys bonds. Ozone is a very reactive allotrope of oxygen and reacts readily with alkenes. Alkenes form organic compounds in which a carbonyl group replaces the multiple carbon-carbon bonds. The outcome of the reaction depends on the type of multiple bonds being oxidized and the workup conditions.
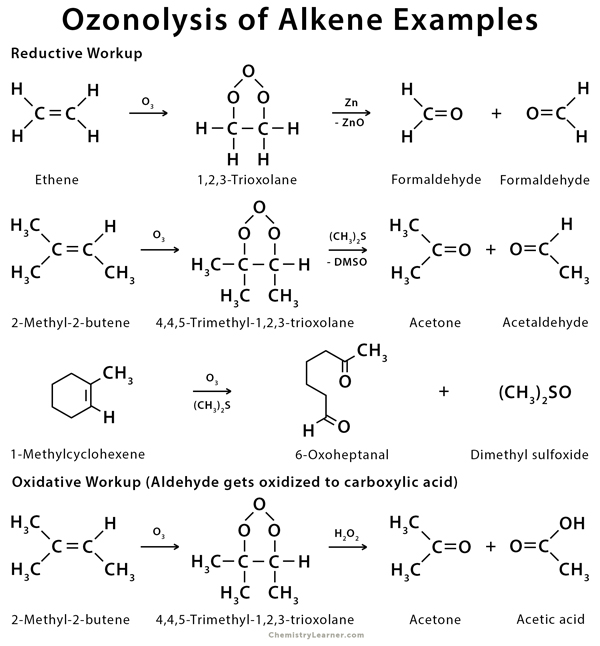
During this reaction, the ozone breaks the C=C bond of alkenes and forms an intermediate compound called ozonide. The ozonide is decomposed into carbonyl compounds by a reducing agent like zinc (Zn) or dimethyl sulfide ((CH3)2S). At the same time, the reducing agent gets oxidized to zinc oxide (ZnO) or dimethyl sulfoxide (DMSO). This process is known as reductive workup and results in aldehyde and ketone. On the other hand, if hydrogen peroxide is used instead of ZnO or (CH3)2S then any aldehyde formed is oxidized to a carboxylic acid. This process is known as oxidative workup [1-4].
The history of ozonolysis goes back to 1840 when German-Swiss chemist Christian Friedrich Schönbein first invented it.
Examples of Ozonolysis of Alkenes
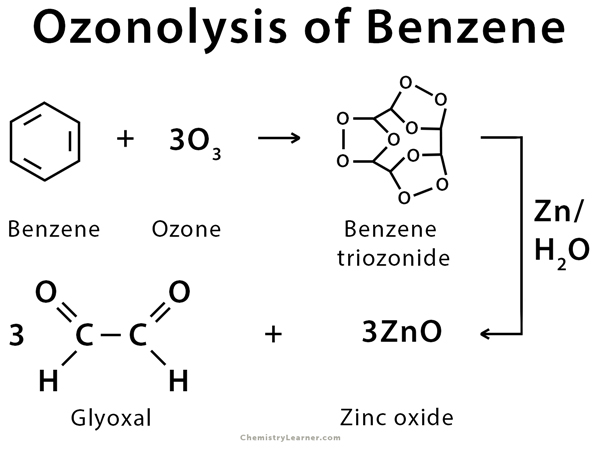
Ozonolysis can be carried out on alkenes like ethene, butene, and pentene and cyclic compounds like cyclopentene and cyclohexene, which are then converted into their corresponding carbonyl compounds. The ozonolysis of benzene forms glyoxal through the formation of benzene triozonide as an intermediate [2-5].
Mechanism of Ozonolysis of Alkenes
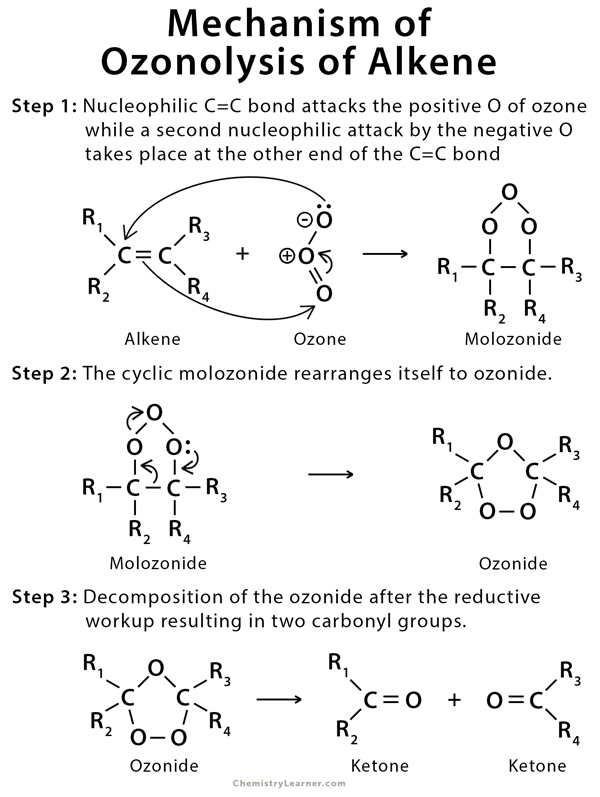
The mechanism proceeds via an oxidative cleavage reaction with ozone through a three-step process. Ozone reacts with an alkene via a cycloaddition reaction to form an unstable 5-membered ring called a molozonide. It starts with a 1,3-dipolar cycloaddition but eventually ends up cleaving pi bonds in an oxidative manner resulting in two carbonyl groups [3,4,6,7].
Ozonolysis of Alkynes
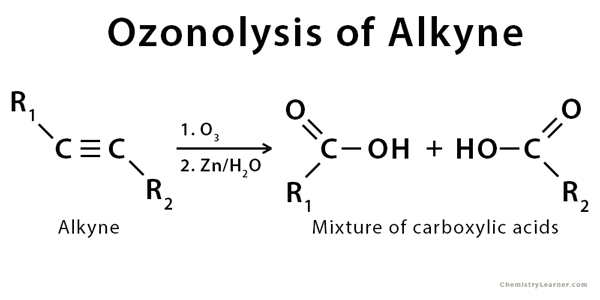
Ozonolysis of alkynes gives an acid anhydride or a diketone. The fragmentation is incomplete in this reaction. A reducing agent is not needed as a simple aqueous workup gives a mixture of carboxylic acids.
Application of Ozonolysis
Ozonolysis is used to determine the position of the double bond in alkenes and triple bonds in alkynes. It has been used extensively in determining the structure of natural products, particularly terpenes, and for synthesizing rare aldehydes and ketones.
References
- Definition – Chem.libretexts.org
- Definition and example – Chem.ucla.edu
- Definition, examples, and mechanism – Chem.ucalgary.ca
- Definition, examples, and mechanism – Ursula.chem.yale.edu
- Example – Organic-chemistry.org
- Mechanism – Chemtube3d.com
- Mechanism – Slideshare.net






Nice
Great work
I need more explanation
May I know what kind of explanation is needed?
How would the mechanism be if the ozonide were reduced by NaBH4 to form alcohols? No other reagents involved.
If ozonolysis is followed by reduction with NaBH₄ alone, the ozonide is reduced to alcohols instead of aldehydes or ketones. NaBH₄ donates hydride ions (H⁻), breaking the oxygen-oxygen bonds in the ozonide and forming hydroxyl (-OH) groups. As a result, internal alkenes yield two secondary alcohols, while terminal alkenes produce a primary and a secondary alcohol. This reaction is less common than standard reductive ozonolysis (Zn/H₂O or Me₂S) but has been studied in certain cases.