Molybdenum
.What is Molybdenum
Molybdenum (pronunciation: meh-LIB-deh-nem) is a shiny, silvery element belonging to the family of transition metals and is represented by the chemical symbol Mo [1, 2, 3]
. A ductile metal with high corrosion resistance, molybdenum reacts easily with other elements to form compounds [2].
It has seven naturally occurring isotopes, out of which 98Mo is the most abundant comprising about 24.39% of all the molybdenum found on Earth [4]. Of the seven naturally occurring isotopes, 100Mo is not considered stable and has a half-life period of 7.8 X 1018 years [4].
Where is Molybdenum Found
Molybdenite (also known as molybdenum disulfide or MoS2) is the principal mineral ore from which the metal is extracted by oxidation roasting and then reducing the resultant molybdenum oxide [1]. A small amount of molybdenum also occurs as a by-product during the production of copper and tungsten [1]. The top 3 molybdenum reserve holding countries are China, the USA, and Chile while the top 3 producers include China, the USA, and Chile [1].
History
Origin of its Name: The name of the element is derived from ‘molybdos’, the Greek word for lead [1, 2].
Who discovered it: The Swedish chemist Peter Jacob Hjelm is the discoverer of molybdenum [1].
When and How was it Discovered
The soft, black molybdenite ore is similar to graphite in appearance and was thus incorrectly regarded as a lead ore until it was analyzed by the German-Swedish chemist Carl Wilhelm Scheele in 1778 [1]. Although he could not identify the substance, his research proved that it was neither graphite nor lead [1].
It was speculated that the mineral ore contained an unknown element but the researchers could not reduce and isolate the metal [1]. They found that the new element could be oxidized and it formed an acid when mixed with water, called molybdic acid (H2MoO4) [1]. In 1781, Peter Jacob Hjelm ground carbon and molybdic acid together with linseed oil and formed a paste [1]. He heated the mixture in a crucible and produced the molybdenum metal [1, 5].
Molybdenum Identification |
|||
| Atomic number | 42 [1] | ||
| CAS number | 7439-98-7 [1] | ||
| Position in the periodic table | Group | Period | Block |
| 6 [1] | 5 [1] | d [1] | |
Properties and Characteristics of Molybdenum
General Properties |
||||||||||||||||
| Relative atomic mass | 95.95 [1] | |||||||||||||||
| Atomic mass | 95.95 amu [1] | |||||||||||||||
| Molar mass | 95.96 g/mol [9, 10] | |||||||||||||||
Physical Properties |
||||||||||||||||
| Color | Silvery-white [1, 5] | |||||||||||||||
| Melting point/freezing point | 2622 °C, 4752 °F [1] | |||||||||||||||
| Boiling point | 4639 °C, 8382 °F [1] | |||||||||||||||
| Density | 10.2 g cm-3 [1] | |||||||||||||||
| State of matter at room temperature (solid/liquid/gas) | Solid [1, 5] | |||||||||||||||
| Hardness | ||||||||||||||||
| – Brinell | 1500 MPa [6] | |||||||||||||||
| – Mohs | 5.5 [6] | |||||||||||||||
| – Vickers | 1530 MPa [6] | |||||||||||||||
| Electrical conductivity | 2X107 S/m [6] | |||||||||||||||
| Thermal (heat) conductivity | 139 W/(m K) [6] | |||||||||||||||
| Specific heat | 251 J kg-1 K-1 [1] | |||||||||||||||
| Bulk modulus | 231 GPa [1] | |||||||||||||||
| Shear modulus | Unknown [1] | |||||||||||||||
| Young’s modulus | Unknown [1] | |||||||||||||||
| Vapor pressure | ||||||||||||||||
| – Temperature (K) | 400 | 600 | 800 | 1000 | 1200 | 1400 | 1600 | 1800 | 2000 | 2200 | 2400 | |||||
| – Pressure (Pa) | – | – | – | – | – | – | 1.83X 10-9 | 4.07X 10-7 | 3.03X 10-5 | 1.02X 10-5 | 1.89X 10-2 | |||||
Chemical Properties |
||||||||||||||||
| Oxidation state/Oxidation number | −2, −1, +1 +2, +3, +4, +5, +6 [1] | |||||||||||||||
| Isotopes | Isotope | Mass | Abundance (%) | Half-life | Mode of decay | |||||||||||
| 92Mo | 91.907 | 14.53 | > 3 X 1017 y | β+-EC | ||||||||||||
| 94Mo | 93.905 | 9.15 | – | – | ||||||||||||
| 95Mo | 94.906 | 15.8 | – | – | ||||||||||||
| 96Mo | 95.905 | 16.67 | – | – | ||||||||||||
| 97Mo | 96.906 | 9.60 | – | – | ||||||||||||
| 98Mo | 97.905 | 24.39 | – | – | ||||||||||||
| 100Mo | 99.907 | 9.82 | 6 X 1020 y | β-β- | ||||||||||||
Atomic Data of Molybdenum (Element 42)
| Valence electrons | 6 [7] | |||||||
| Quantum numbers | ||||||||
| – n | 4 [7] | |||||||
| – ℓ | 2 [7] | |||||||
| – mℓ | 2 [7] | |||||||
| – ms | +1/2 [7] | |||||||
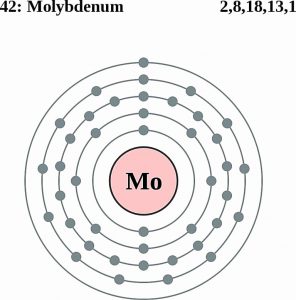
| Electron configuration (noble gas configuration) | [Kr] 4d55s1 [1] | |||||||
| Atomic structure | ||||||||
| – Number of electrons | 42 [5] | |||||||
| – Number of neutrons | 54 [5] | |||||||
| – Number of protons | 42 [5] | |||||||
| Radius of Atom | ||||||||
| – Atomic radius | 2.17 Å [1] | |||||||
| – Covalent radius | 1.46 Å [1] | |||||||
| Electronegativity (Pauling-scale) | 2.16 [1] | |||||||
| Electron affinity | 72.171 kJ mol-1 [1] | |||||||
| Bonding energy | 6.8 eV (656.098 kJ mol-1) [11] | |||||||
| Ionization energy (kJ mol-1) | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th |
| 684.316 | 1559.2 | 2617.65 | 4476.9 | 5257.49 | 6640.85 | 12124.73 | 13855.3 | |
Molybdenum Electron Configuration (Bohr Model)
What is Molybdenum Used for
Uses in Industry
- Since it has a high melting point, it is used to produce a gray powder, which is compressed at very high pressure for manufacturing heat tubes, vacuum tubes, and X-ray tubes [1].
- The element 42 is used in making alloys including steel for increasing its hardness, strength, resistance to corrosion, and electrical conductivity [1, 5].
- Molybdenum alloys are used in aircraft engines, power generation turbines, and nuclear power plants, as well as in heating elements like saw blades and drills [1, 5].
- Molybdic oxide, molybdates, and molybdenum disulfide are used in high-performance lubricant formulations [1, 8].
- Because molybdenum can withstand high temperatures, it is considered the right material for producing glass melting electrodes [5].
- Used as a catalyst in the petroleum industry, it helps in removing organic sulfur in gas and coal liquefaction processes [1, 5].
Biological Role in Plants, Animals, and Humans
- Molybdenum is an important component in various enzymes including nitrogenase, allowing nitrogen-fixing bacteria to take up nitrogen from the air, making it available to the plants [1, 5].
- It is present in plants and animals in trace amounts, helping in synthesizing and utilizing proteins, as well as other metabolic processes [2].
- It acts as a catalyst in the human body for producing enzymes and breaking down amino acids [2].
Role in Scientific Research
- Although molybdenum is currently abundant in the ocean, it was much less in the past. Thus, it is a clear indicator of ancient oceanic chemistry and helps researchers estimate the amount of oxygen that might have been present during a certain period [2].
Molybdenum Toxicity
Although acute molybdenum toxicity has not been reported in humans, animal studies have indicated that high levels of element 42 may interfere with the body’s ability to absorb copper and produce copper deficiency [12]. Mining or metal workers regularly exposed to molybdenum dusts and fumes may suffer from headache, fatigue, weakness, tremors, sweating, dizziness, weight loss, joint and muscle pain, and loss of appetite [13]. According to the NIOSH, the IDLH (immediately dangerous to life and health) for molybdenum compounds is at 5000 mg/m3 [14].
Interesting Facts
- Molybdenum does not react with water or oxygen at room temperatures [5].
- Its oxide is soluble in alkaline water and forms molybdate salts [5].
- When it occurs in compounds, it commonly exists in its oxidation states (IV) and (VI) [5].
- Instead of tungsten, Mo is often used in steel alloys because it has the same metallic effect despite having half the density and atomic weight as tungsten [2].
- Big Bertha, the 47 ton super-heavy artillery gun used by Germany in World War I and II, had molybdenum as one of the constituent element of its steel [2].
- The element is graphically represented by a valve wheel, indicating its use in boilers and valves [1].
- In the US, supplements are prescribed to those who do not get enough molybdenum through their diet [15].
Price of Molybdenum
The cost of pure molybdenum is about $0.44 per gram [5].
- References
- http://www.rsc.org/periodic-table/element/42/molybdenum
- https://www.livescience.com/34687-molybdenum.html
- https://education.jlab.org/itselemental/ele042.html
- https://education.jlab.org/itselemental/iso042.html
- https://www.chemicool.com/elements/molybdenum.html
- http://periodictable.com/Elements/042/data.html
- http://chemistry-reference.com/q_elements.asp?Symbol=Mo
- https://www.imoa.info/molybdenum/molybdenum-products.php
- https://www.webqc.org/molecular-weight-of-Mo.html
- https://webbook.nist.gov/cgi/inchi/InChI%3D1S/Mo
- Ods.od.nih.gov
- https://www.msdvetmanual.com/toxicology/molybdenum-poisoning/overview-of-molybdenum-poisoning
- https://web.archive.org/web/20070919204536/http://rais.ornl.gov/tox/profiles/molybdenum_f_V1.shtml
- https://www.cdc.gov/niosh/npg/npgd0433.html
- https://articles.mercola.com/sites/articles/archive/2016/10/17/molybdenum.aspx