Mannich Reaction
Definition: What is Mannich Reaction?
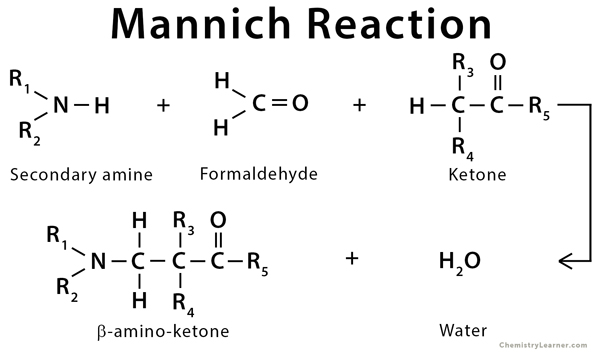
The Mannich reaction is an organic reaction, which is used to convert a primary or secondary amine and two carbonyl compounds (one non-enolizable and one enolizable) to a β-amino carbonyl compound. Usually, an acid or base is used as a catalyst. Generally, formaldehyde is used as the non-enolizable carbonyl compound, and the product is known as Mannich base or product. Reactions between aldimines and α-methylene carbonyls are also considered as Mannich reactions because these imines are formed between amines and aldehydes [1-3].
The history of this reaction goes back to 1912 when a German chemist Carl Mannich discovered it.
Examples of Mannich Reaction
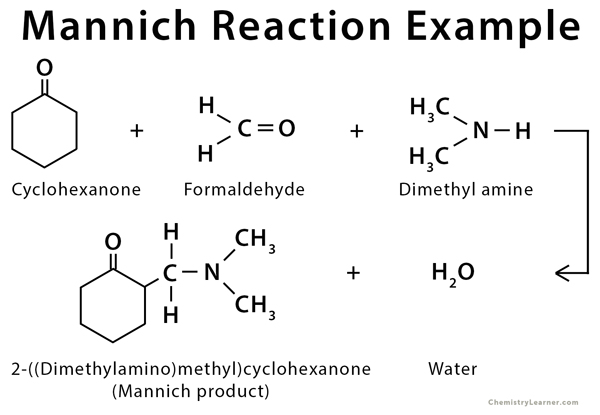
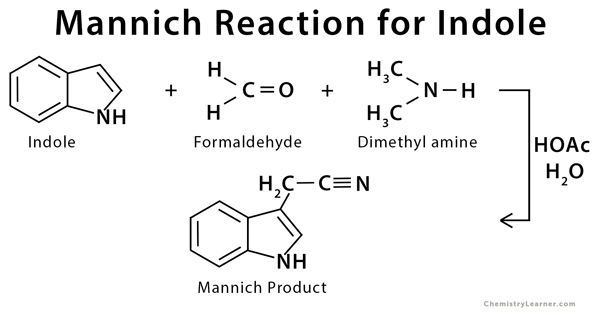
Mannich Reaction can be used with cyclohexanone and dimethylamine to give the desired Mannich product. Indole undergoes Mannich reaction through substitution by elimination and conjugate addition [4-5].
Mechanism of Mannich Reaction
The mechanism of Mannich reaction involves two steps.
Applications of Mannich Reaction
Mannich reaction finds its application in many areas of organic chemistry. It is used to synthesize natural compounds like peptides, nucleotides, antibiotics, and alkaloids [1].
References
- Definition – libretexts.org
- Definition – Organic-reaction.com
- Definition – ac.in
- Example – com
- Example – com
- Mechanism – Name-reaction.com
- Mechanism – Organic-chemistry.org






Thanks for this it’s helping me in my research