Lucas Reagent
Lucas reagent is a solution of anhydrous zinc chloride (Lewis acid) in concentrated hydrochloric acid. It is used as a reagent to test alcohols and classify them in accordance to their reactivity. The reaction is a substitution reaction where the chloride of the zinc chloride gets replaced by the hydroxyl group of the alcohol.
The reactivity of the alcohol with Lucas Reagent is measured by the degree of turbidity which may vary from colorless to turbid. The formation of turbid solution happens due to the formation of chloroalkane.
This experiment was done in 1930. Since then, it is utilized as a standard technique in organic chemistry experiments. But now this method is not widely used as large number of spectroscopic and chromatographic analytical methods have replaced it.
Lucas Reagent Formula
Lucas reagent is basically a solution which is formed by the combination of HCl and ZnCl2
Lucas Test
This test is more often used to categorize the different types of alcohols based on the time taken to form a turbid solution or precipitation using the Lucas Reagent namely:
- Primary alcohol: Here no visible reaction is observed and the solution remains colorless e.g. 1-Pentanol
- Secondary alcohol: Here the solution turns turbid or cloudy in 5-20 minutes with slight heating e.g. 2-Pentanol
- Tertiary alcohol: Here the solution turns turbid or cloudy rapidly with the formation of two separate layers at room temperature e.g. 2-Methyl-2-butanol
In Lucas test, zinc chloride acts as a catalyst. The classification of the alcohols is usually done based on the difference in reaction with concentrated hydrochloric acid. A simple reaction is given below:
ZnCl2
ROH + HCl—>RCl + H2O
The tertiary alcohol undergoes the most stable reaction and the primary alcohol undergoes the least stable reaction. This test can be conducted only with those alcohols which are soluble in Lucas reagent and with lower molecular weight. Alcohols generally with more than six carbon atoms cannot be tested.
Lucas Reagent Preparation
The Lucas reagent can be prepared by the following steps:
- Pour the concentrated HCl into a 50 ml graduated cylinder. Measure out 47 ml of concentrated HCl and pour it into the 100 ml beaker
- Place the 100 ml beaker in the ice bath to absorb the heat generated during the dissolution of the ZnCl2
- Weigh out 62.5 g of anhydrous ZnCl2 and allow it to dry in an oven for at least two hours. Cool the anhydrous ZnCl2 in a dessicator to prevent air contact.
- Add the ZnCl2 to the hydrochloric acid in the beaker slowly to avoid the mixture overflowing the sides of the small beaker.
- Stir the mixture until the ZnCl2 dissolves completely to form the Lucas Reagent. Store the reagent in a cool, dry place for later use.
Lucas Reagent Mechanism
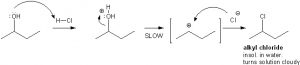
The reaction which normally occurs is a SN1 nucleophilic substitution which is a two steps reaction. Alcohols which have a capability to form carbocation intermediates exhibit this reaction. Only secondary and tertiary alcohols exhibit the SN1 nucleophilic mechanism.
The two steps which are generally followed in this reaction:
- In the first step the proton (H+) from Hydrochloric acid (HCl) will protonate the OH– group of the alcohol. Water (H2O) attached to the carbon is a weaker nucleophile than Cl (Chloride). Thus nucleophile Cl– replaces the H2O group forming a carbocation as its present in excess.
- In the second step the Cl– attacks the carbocation and thus forms alkyl chloride.
Here the first step is generally the slowest step and is the rate-determining step. As the tertiary carbocation is much stabilized, they are the ones that undergo reaction and form a turbid solution. The opposite of it happens in the case of primary alcohols.
Thus on the basis of the reactions and the rate of all the three reactions, we can not only measure the reactivity but also characterize the three groups of alcohols.
Lucas Reagent MSDS
Lucas reagent is highly toxic and corrosive and should be handled carefully while conducting the experiment. The toxicity and corrosiveness arise as a result of the constituents.
- Hydrochloric acid can irritate the skin. The vapors should not be inhaled as they might affect the respiratory system.
- Zinc Chloride is highly corrosive and may cause damage to the skin and the respiratory system.
Apart from the constituents, the vapors of the alcohol are slightly irritating to the eyes and nose. It should not be inhaled as it may turn fatal.
- References
- http://en.wikipedia.org/wiki/Lucas’_reagent
- http://en.wikipedia.org/wiki/Zinc_chloride#Safety_considerations
- http://www.mendelset.com/sites/default/files/userfiles/1/uploaded_images/alcohols04.png
- http://academics.wellesley.edu/Chemistry/chem211lab/Orgo_Lab_Manual/Appendix/ClassificationTests/alcohol.html#Lucas
- http://organicchem.org/oc2web/lab/exp/oxid/lucas.pdf





Helpfull information .. Thank You for providing the details
Thank you…????????
Thanks????
thank youuuuuuu i finally understand it now 😀
Thankss .. Finally i cleared my concept..
Clear and straightforward, thank you.
Thank you *-*
Thanks alot. I really appreciate. I will like to make one request_your facebook username
thanks
satisfied
Thanku for clearing my topic
Thnks
nw i got it…
Thankyou.
Thank you so much I understand this thing now
one. question: an example of a primary alcohol whose response to lucas test is as fast as a tertiary alcohol
Any benzyl or allyl alcohol
wao thanks so much now I understand
Thank you ^_^
THANKS…
why doesn’t nitro phenol react with lucas reagent?
Nitro phenol does not react with Lucas reagent because it is a primary alcohol.
why anhydrous zinc chloride is used in lucus reagent????????????
Anhydrous zinc chloride is used so that it absorbs water and acts as a dehydrating agent.
Is really educative
very good
Thanks
THANKS
Thanks a lot……
Thanks
How much temperature should be when it keeps the oven
Around 100 degrees C.
this is very helpful and easy to understand
Thanks you
Thanks. It’s crystal clear now.
I don’t understand
What exactly you don’t understand?
Uses of Lucas reagent
As mentioned in the first paragraph, it is used to test alcohols and classify them in accordance to their reactivity.
Simplistic explanation that turned what was rocket science to me into an enjoyable and easy to follow process. Thank you.