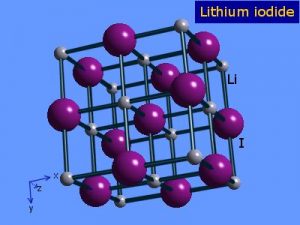
Lithium Iodide
Lithium iodide, represented by the chemical formula LiI, is an inorganic compound with constituent elements lithium and iodine [1]. It can be easily identified because exposure to air changes its color from white to yellow due to the formation of iodine by oxidation of iodide [2]. LiI can exist in different hydrate forms, including monohydrate, dehydrate, and trihydrate [3].
How is Lithium Iodide Prepared
Lithium iodide can be synthesized by combining lithium sulfide and strontium iodide, which can be represented by the double decomposition reaction given below [5]:
Li2S + SrI2 → SrS + 2LiI
Another method for anhydrous lithium iodide preparation involves the following reaction of lithium aluminum hydride with iodine in a diethyl-ether medium [6]:
LiAlH4 + 2I2 → 2H2 + AlI3 + LiI
When LiI is dissolved in water, the ions are stabilized by water molecules, giving off energy that is greater than the lattice energy of LiI. The dissolution of lithium iodide is thus exothermic.
Properties and Characteristics of Lithium Iodide
General Properties |
|
| Molar Mass/Molecular Weight | 133.844 g/mol [1] |
Physical Properties |
|
| Color and Appearance | White crystalline solid [7] |
| Melting Point | 446-469 °C, 834.8-876.2 °F [4, 7] |
| Boiling Point | 1170-1190 °C, 2138-2174 °F [4] |
| Density | 4.08 g cm-3 [7] |
| State of matter at room temperature (solid/liquid/gas) | Solid [7] |
| Solubility | Soluble in water, methanol, acetone, and alcohol; very soluble in an aqueous solution of ammonia [4] |
| Solubility in Water | 1510 g/L (at 0 °C), 1670 g/L (at 25 °C), 4330 g/L (at 100 °C) |
| Heat Capacity (C) | 54.4 J/mol K or 0.381 J/g K |
| Magnetic Susceptibility (χ) | -50 X 10-6 cm3/mol |
Chemical Properties |
|
| pH | N/A |
| Acid or base | N/A |
Atomic Properties |
|
| Crystal Structure | Face-centered cubic lattice |
Uses
- Iodide-based electrolytes containing lithium are potentially used in high-temperature batteries and long-life batteries, including artificial pacemakers [8, 9].
- Lithium iodide crystals are grown for use as scintillation detectors of neutrons [10].
- It can be used for cleaving carbon-oxygen bonds, such as the conversion of methyl esters into carboxylic acid.
Is it Safe
Overexposure or repeated exposure can cause skin irritation [1]. Contact with eyes can cause serious eye damage [1].
- References
- Lithium Iodide – Pubchem.ncbi.nlm.nih.gov
- Lithium iodide – Chem.libretexts.org
- Higher hydrates of lithium chloride, lithium bromide and lithium iodide – Scripts.iucr.org
- Lithium iodide – Chemspider.com
- Chemical Equation Balancer – Chemicalaid.com
- Preparation of anhydrous lithium iodide – Inis.iaea.org
- Lithium Iodide – Americanelements.com
- All-Lithium, Iodide-Based, Low-Melting Electrolytes for High-Temperature Batteries – Ecst.ecsdl.org
- Trends in Cardiac Pacemakers – Ncbi.nlm.ih.gov
- Some lithium iodide phosphors for slow neutron detection – Iopscience.iop.org