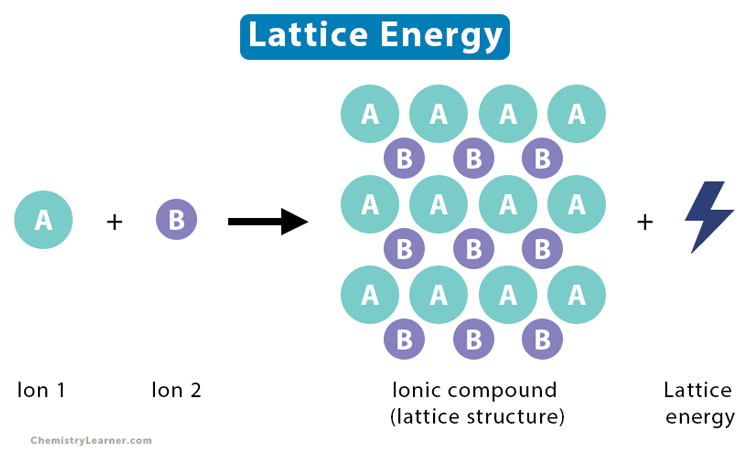
Lattice Energy
What is Lattice Energy
The lattice energy is the energy change occurring when one mole of a solid ionic compound forms in its gaseous state. It also refers to the energy required to disassociate one mole of a solid compound into its component gaseous ions. Lattice energy can be released (exothermic) or absorbed (endothermic) depending on whether the compound forms or disassociates. It measures the forces that bind the atoms together in a crystal lattice. The importance of lattice energy is that it gives information on several properties like volatility, solubility, and hardness [1-4].
General Reaction for Disassociation of a Solid
AB (s) → Am+ (g) + Bn- (g)
How to Find Lattice Energy of Compounds
The lattice energy magnitude of an ionic crystal can be determined from the following equation derived from Coulomb’s law [2,4].
|ΔHlattice| = k Q1Q2/ro
Where,
ΔHlattice: lattice energy
Q1 and Q2: charges on the ions
ro: interionic distance
k: proportionality constant
Unit: kJ/mole
Lattice energy can be calculated using electrostatics or estimated from the Born-Haber cycle.
Factors Affecting Lattice Energy [1,3,6]
1. Ionic radius: As the ionic radius increases, the lattice energy decreases. In other words, the bond between opposite ions is strongest when the ions are small. The following table shows the lattice energy values (in kJ/mol) for the ionic bond formed between alkali metals and halogens. It is clear that the bond between Li+ and F– (LiF) has the highest lattice energy and that between Cs+ and I– (CsI) has the lowest.
| F– | Cl– | Br– | I– | |
| Li+ | 1036 | 853 | 807 | 757 |
| Na+ | 923 | 787 | 747 | 704 |
| K+ | 821 | 715 | 682 | 649 |
| Rb+ | 785 | 689 | 660 | 630 |
| Cs+ | 740 | 659 | 631 | 604 |
2. Atomic charge: As the atomic charge increases, the lattice energy increases. In other words, the ionic bond becomes stronger as the charge on the ions becomes large. The lattice energy is proportional to the product of the two ionic charges (ΔHlattice ∝ |Q1Q2|). The following table shows the lattice energies for salts of OH– and O2-. It is clear that the bond between Na+ and OH– (NaOH) has the smallest lattice energy, and that between Al3+ and O2- (Al2O3) has the greatest.
| OH– | O2- | |
| Na+ | 900 | 2481 |
| Mg2+ | 3006 | 3791 |
| Al3+ | 5627 | 15916 |
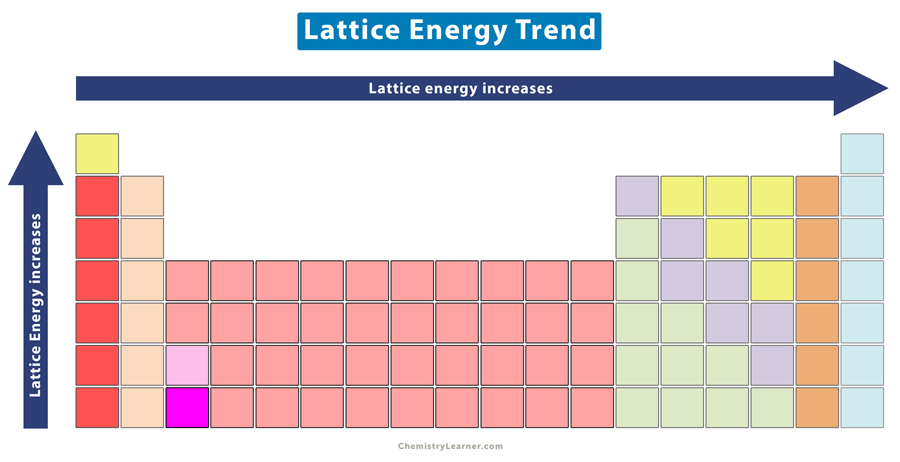
Lattice Energy Trend in Periodic Table
From the above tables, one can observe that the lattice energy increases with atomic charge and decreases with ionic radius. Across a period, the atomic charge increases, and down a group, the ionic radius increases. Hence, the lattice energy increases from left to right across a period and decreases from top to bottom down a group [4].
Lattice Energy Examples [1-6]
1. Sodium Chloride (NaCl)
When sodium ion (Na+) combines with chloride ion (Cl–), sodium chloride (NaCl) crystal forms, and 787.3 kJ of energy is released.
Na+ (g) + Cl– (g) → NaCl (s) ΔHlattice = -787.3 kJ/mol
2. Calcium Oxide (CaO)
When calcium ion (Ca2+) combines with oxide ion (O2-), calcium oxide (CaO) crystal forms, and 3414 kJ of energy is released.
Ca2+ (g) + O2- (g) → CaO (s) ΔHlattice = -3414 kJ/mol
3. Calcium Chloride (CaCl2)
When calcium ion (Ca2+) combines with chloride ion (Cl–), calcium chloride (CaCl2) crystal forms, and 2195 kJ of energy is released.
Ca2+ (g) + 2 Cl– (g) → CaCl2 (s) ΔHlattice = -2195 kJ/mol
4. Magnesium Oxide (MgO)
When magnesium ion (Mg2+) combines with oxide ion (O2-), magnesium oxide (MgO) crystal forms, and 3795 kJ of energy is released.
Mg2+ (g) + O2- (g) → MgO (s) ΔHlattice = -3791 kJ/mol
5. Magnesium Chloride (MgCl2)
When magnesium ion (Mg2+) combines with chloride ion (Cl–), magnesium chloride (MgCl2) crystal forms, and 2450 kJ of energy is released.
Mg2+ (g) + 2 Cl– (g) → MgCl2 (s) ΔHlattice = -2540 kJ/mol
The lattice energy is related to the physical properties of the ionic compound. For example, it can predict the solubility of ionic compounds.
Lattice Energy and Solubility
The lattice energy of ionic salts indicates their solubility in water because it represents the energy needed to separate ions in solution. Salts with higher lattice energy are insoluble in water than lower ones. For example, from the above table, one can find that sodium hydroxide (NaOH) has lower lattice energy than magnesium hydroxide (Mg(OH)2), which is lower than aluminum hydroxide (Al(OH)3). Therefore, NaOH can easily dissolve in water, Mg(OH)2 is sparingly soluble, and Al(OH)3 is insoluble.
FAQs
Ans. The difference between lattice energy and lattice enthalpy is the conditions under which they are calculated.
Ans. The difference between lattice energy and solvation energy is that lattice energy gives the energy change during the formation or disassociation of an ionic compound. The solvation energy gives the energy absorbed or liberated when dissolving a solute in a solvent.




Thanks a lot, this is so helpful… I just wanted one but you gave me all thanks and thanks again..????????????