Keto-Enol Tautomerism
Definition: What is Keto-Enol Tautomerism?
Tautomers are isomers of a compound that differ only in the position of the proton and electrons. The carbon skeleton of the compound remains unchanged. A reaction that involves a simple proton transfer through an intramolecular mechanism is called tautomerism. In keto-enol tautomerism, a carbonyl double bond is broken, and an alkene double bond is formed. Keto-enol tautomerism refers to a chemical equilibrium between the keto form (carbonyl structure containing α-hydrogen) and the enol form (a double bond adjacent to an alcohol, -C=C-OH) of a compound. The enol and keto forms are said to be tautomers of each other. It is a comprehensive process that is acid or base-catalyzed. Typically, the keto form of the compound is more thermodynamically stable and highly favored. However, in some instances, the enol form can be more stable. Its stability depends upon factors like aromaticity, hydrogen bonding, solvent, conjugation, and substitution [1-5].
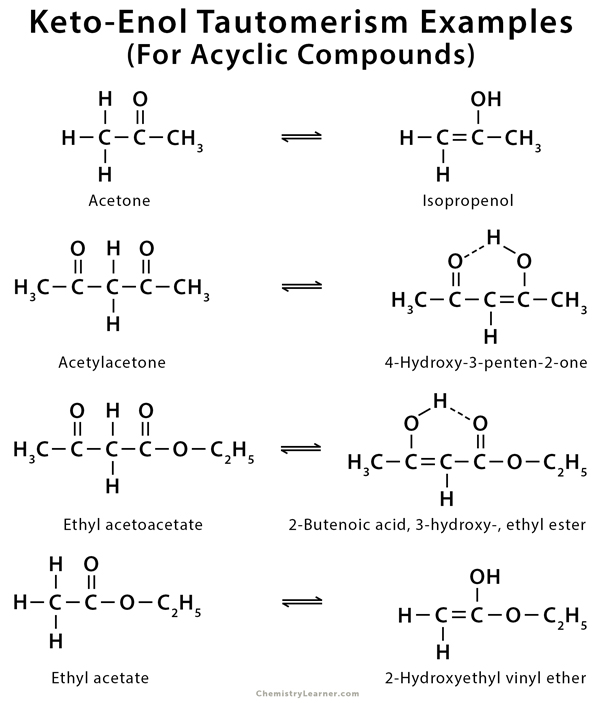
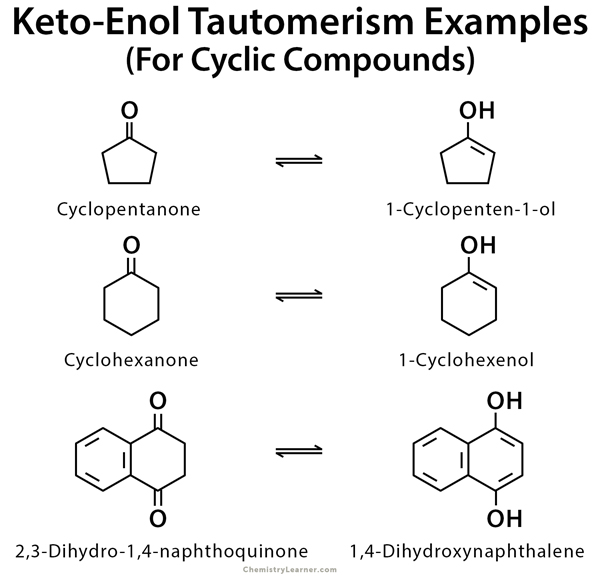
Examples of Keto-Enol tautomerism
As mentioned before, keto-enol tautomerism takes place in carbonyl compounds having alpha hydrogen. These include acetone, ethyl acetate, and ethyl acetoacetate [1-9].
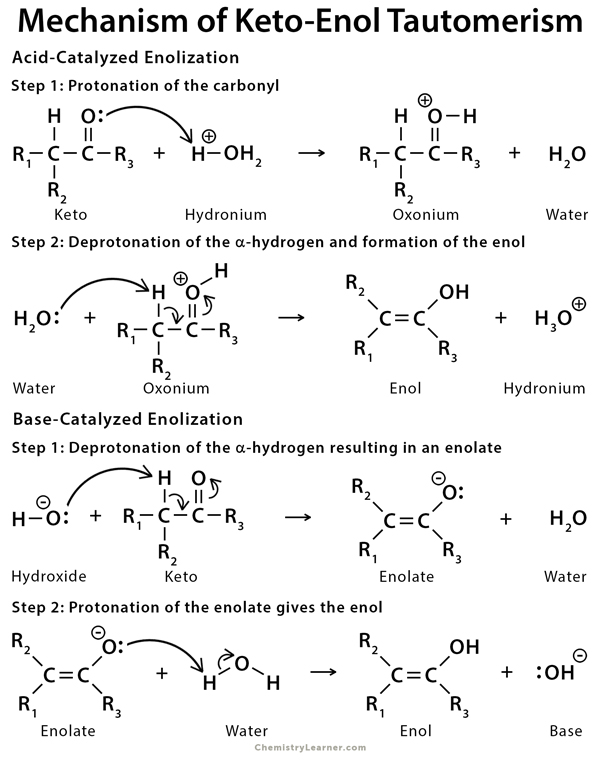
Mechanism of Keto-Enol Tautomerism
In acidic conditions, the mechanism for keto-enol tautomerism involves protonation of the carbonyl oxygen, followed by deprotonation of the alpha hydrogen. In basic conditions, the mechanism for keto-enol tautomerism involves deprotonation of the alpha hydrogen and formation of a negatively charged enolate intermediate that is protonated on oxygen to form the enol [4,5,10].
FAQ
Ans. Tautomerization is a process where single bonds are broken, and atoms are rearranged to give a different structure. In resonance structure, atoms are rearranged, but no bond is broken.
Ans. The keto form is more thermodynamically stable than the enol form because it has higher bond energy. The sum of the C-H, C-C, and C=O bond energies is higher than that of the C=C, C-O, and O-H bonds in enol.
Ans. The enol form of acetylacetone is reported to be more stable, and an internal hydrogen bond stabilizes it. The formation of this hydrogen bond is further facilitated by the six-membered planar structure of the enol-carbonyl resonance system. Besides, the lone pair of electrons on the enol oxygen can delocalize across the five atoms to the electronegative carbonyl oxygen.
References
- Definition and example – Chem.ox.ac.uk
- Definition and example – Chem.ucalgary.ca
- Definition and example – Epgp.inflibnet.ac.in
- Definition, example, and mechanism – Chem-net.blogspot.com
- Definition, example, and mechanism – Organicchemistrytutor.com
- Example – Chem.libretexts.org
- Example – Ochempal.org
- Example – Researchgate.net
- Example – Slideshare.net
- Mechanism – Chemistryscore.com
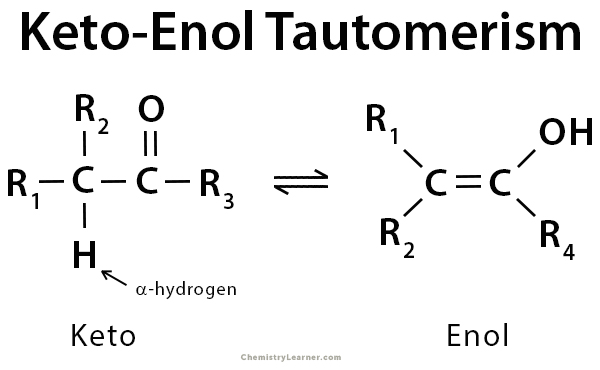





There is a missing double bond (C=C) in the first image on this page.
https://www.chemistrylearner.com/keto-enol-tautomerism.html
Thank you. We’ll make the change.