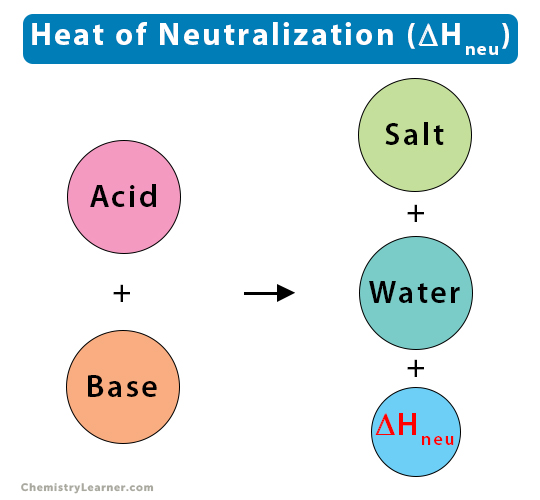
Heat of Neutralization
The heat or enthalpy of neutralization is the change in enthalpy occurring when an aqueous acid reacts with an aqueous base to form salt and one mole of water under standard conditions. This type of reaction is known as a neutralization reaction, and the calculated enthalpy is a particular case of the enthalpy of reaction. The enthalpy change for a neutralization reaction is always negative, indicating that heat is always liberated (exothermic reaction) [1-4].
Reaction between Strong Acid and Strong Base
Aqueous solutions of strong acids and bases completely ionize to give their constituent ions. For example, hydrochloric acid (HCl) ionizes into hydrogen ions (H+) and chloride ions (Cl–) ions [1,2].
HCl (aq.) ⇄ H+ (aq.) + Cl– (aq.)
Also, sodium hydroxide (NaOH) completely ionizes to give sodium ions (Na+) and hydroxide ions (OH– ) ions.
NaOH (aq.) ⇄ Na+ (aq.) + OH– (aq.)
The H+ ions and OH– ions combine to form water (H2O).
2 H+ (aq.) + OH– (aq.) ⇄ H2O (l)
Therefore, the overall reaction between HCl and NaOH is as follows:
HCl (aq.) + NaOH (aq.) ⇄ Na+ (aq.) + Cl– (aq.) + H2O (l)
The reaction between any strong acid and a strong base directly involves H+ ions combining with OH– ions. The anion from the acid (Na+) and the cation from the base (Cl–) are spectator ions. It has been experimentally observed that the heat of neutralization for the above reaction is – 57.9 kJ·mol-1.
For any neutralization reaction between a strong acid and a strong base, the heat of neutralization lies between -57 and – 58 kJ·mol-1. This constancy of heat of neutralization values can be explained by ionic theory.
Reaction between Weak Acid and Strong or Weak Base
Unlike strong acids, weak acids do not ionize completely. They partially disassociate. Weak acids require energy to break the bonds and produce hydrogen ions. It means that the heat of neutralization involves other terms like enthalpy changes for ionizing the acid and H+ reacting with OH–. Therefore, for reactions involving weak acids, the enthalpy of neutralization is less than that of strong acids [1,2].
Let us take a few examples to illustrate this fact. Suppose sodium hydroxide is neutralized by acetic acid (CH3COOH). The reaction is as follows:
CH3COOH (aq.) + NaOH (aq.) ⇄ Na+ (aq.) + CH3COO– (aq.) + H2O (l)
The heat of neutralization for the above reaction is – 56.1 kJ·mol-1. This value is less negative than the HCl and NaOH reaction. It is because 1.8 kJ of extra energy is absorbed for every mole of acetic acid deprotonated.
Suppose sodium hydroxide is neutralized by hydrocyanic acid (HCN). The reaction is as follows:
HCN (aq.) + NaOH (aq.) ⇄ NaCN (aq.) + H2O (l)
The enthalpy of neutralization is only – 11.7 kJ·mol-1. A large amount of energy is required to ionize HCN.
How to Calculate Heat of Neutralization
Suppose specific volumes of the acid and base react in a calorimeter and liberate heat. The heat gained by the resultant solution can be calculated using the following formula [4]:
Qsoln = mcΔT
Where
Q: Heat of the resultant solution
m: Mass of the resultant solution
c: Specific heat of the resultant solution
ΔT: Change in temperature
Since the reaction takes place in a calorimeter, the heat gained by the solution equals the heat lost during the neutralization reaction. In other words,
Qrxn = – Qsoln
The heat of neutralization is then given by
Qneu = Qrxn/ number of moles of the limiting reagent
Example Problem
Equal volumes, 40.0 mL, of 2.0 M hydrochloric acid and 2.0 M sodium hydroxide solutions at an initial temperature of 25.0°C react in a calorimeter. The resultant solution records a temperature of 38.9°C. What is the heat of neutralization?
Solution
Let us rewrite the reaction.
HCl (aq.) + NaOH (aq.) ⇄ NaCl(aq.) + H2O (l)
Since these are aqueous solutions, they consist mainly of water. We can assume that the densities and the specific heat of the solutions are approximately 1.0 g/ml and 4.18 J/g°C, respectively.
Mass of HCl = density x volume = 1.0 g/mL x 40.0 mL = 40 g = Mass of NaOH
Total mass (m) = 2 x 40 g = 80 g
Change in temperature (ΔT) = 38.9 ˚C – 25 ˚C = 13.9 ˚C
c = 4.18 J/g°C
Therefore, the heat gained by the solution is
Qsoln = 80 g x 4.18 J/g°C x 13.9 ˚C = 4648 J
The heat of reaction is
Qrxn = – 4648 J
Since equal concentrations of HCl and NaOH react, no limiting reagent exists. Let us calculate the number of moles of HCl in the reaction.
Volume of HCl = 40 mL = 0.040 L
Concentration of HCl = 2.0 M/L
Number of moles of HCl (n) = 2.0 M/L x 0.040 L = 0.080 M
Therefore, the heat of neutralization is
Qneu = – 4648 J / 0.080 M = – 58102 J/M or 58.1 kJ/mol



Dear readers,
i read your publication about the heat of neutralization in order to get to know why we are only using the mole of HCl an not NaOH to calculate the quotient of Qand n, although both of the educts are getting neutralized. It would be so nice, if you explained it to me.
Greatings
J. Risse
Equal concentration of both were used. No limiting reagent.
Pls send a video of this experiment and the calculations
We do not make videos. We write articles only.