Grignard Reagent
What is Grignard Reagent?
Grignard reagent is an organic derivative of magnesium which is given by the formula RMgX, where X is a halogen and R is an alkyl or aryl group. Grignard reagents are used to create new compounds by joining two alkyl or aryl groups resulting in the formation of new C-C bonds and new functional groups as well [1].
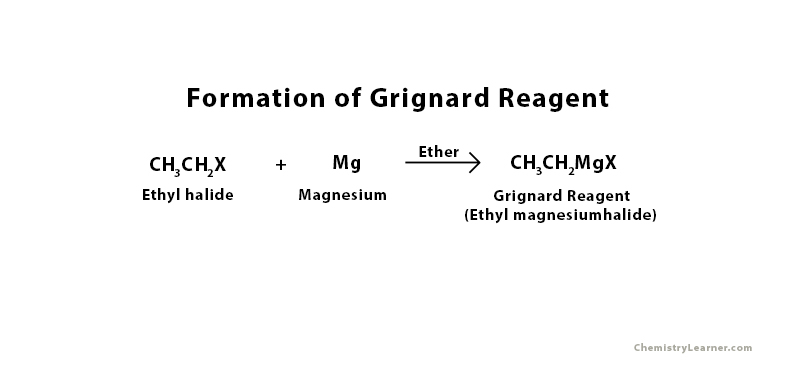
Formation of Grignard Reagent
In order to synthesize Grignard reagent, the following materials should be present in the reaction – magnesium, an alkyl or aryl compound, and a halogen. Typically, bromine and iodine are used as halogens because they combine well with alkyl and aryl groups to form organic halide in the presence of ether. When organic halide reacts with magnesium, the final product is organometallic halide or Grignard reagent [2]. The reaction must be kept dry because the reagent has a tendency to react with water.
Consider the formation of ethylmagnesium halide. In order to form this compound, a small amount of magnesium is mixed with ethyl halide in a flask containing diethyl ether. Diethyl ether is a suitable solvent if the formation of Grignard reagents because it is nonpolar and does not chemically react with the reagent. Fitted with a reflex condenser, the flask is then warmed in a water bath for 20 to 30 minutes [3].
Mechanism of Formation of Grignard Reagents
The mechanism of formation of Grignard reagents takes place through free radical reaction. Unlike other free radical reactions, this mechanism does not involve chain generation or radical chain. Movement of single electrons is a common feature in the formation [4, 5].
Reactivity of Grignard Reagents
Grignard reagents are highly reactive, especially to polar compounds. For example, they can attack the proton (H+ ions) in water and alcohol, the former is strongly covalently bonded with oxygen (O). This is the basis for Grignard reaction. On the other hand, Grignard reagent is also a reducing agent since it can behave as a hydride donor [6].
Applications of Grignard Reagents
Like all organometallic compounds, Grignard reagents have several applications, particularly, in industry. A major application is in Grignard reaction in which the reagents undergo chemical reactions to form several industrial compounds. They have also been used in the synthesis of several natural products [7].
Turbo Grignard Reagent
The addition of one molecule of lithium Chloride (LiCl) during the formation of Grignard reagent enables better insertion of magnesium and superior metal-halogen exchange. The compound RMgCl-LiCl is known as turbo Grignard reagent.
- References
- Definition of Grignard reagent – Britannica.com
- Formation of Grignard reagent – Study.com
- Formation of ethylmagnesium bromide – Chemguide.co.uk
- Mechanism of formation of Grignard reagent –Research.cm.utexas.edu
- Mechanism of formation of Grignard reagent –Chemistry.stackexchange.com
- Grignard reagent as a reducing agent – Drmarkforeman.wordpress.com
- Applications of Grignard reagents – Alfa.com