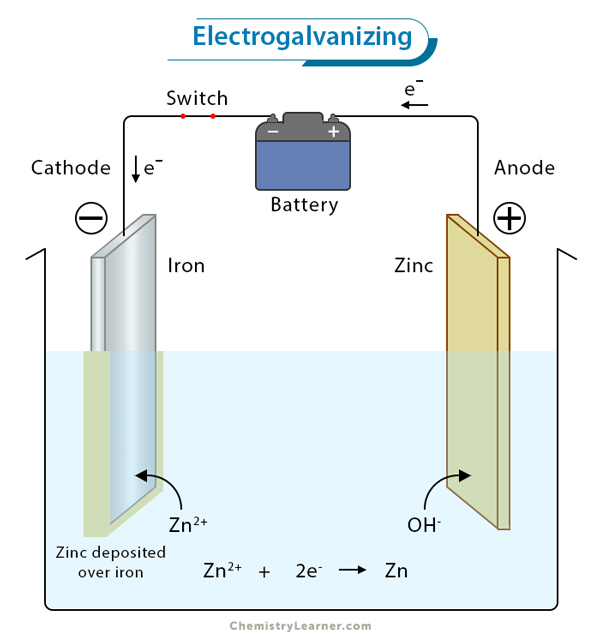
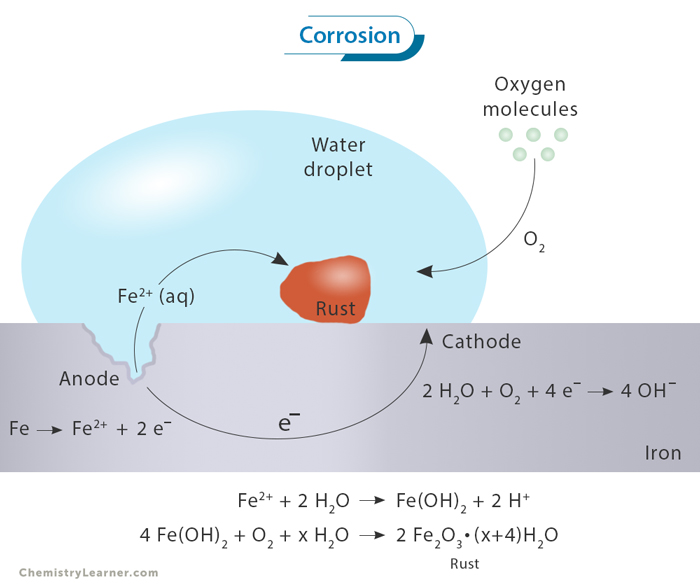
Gravimetric Analysis
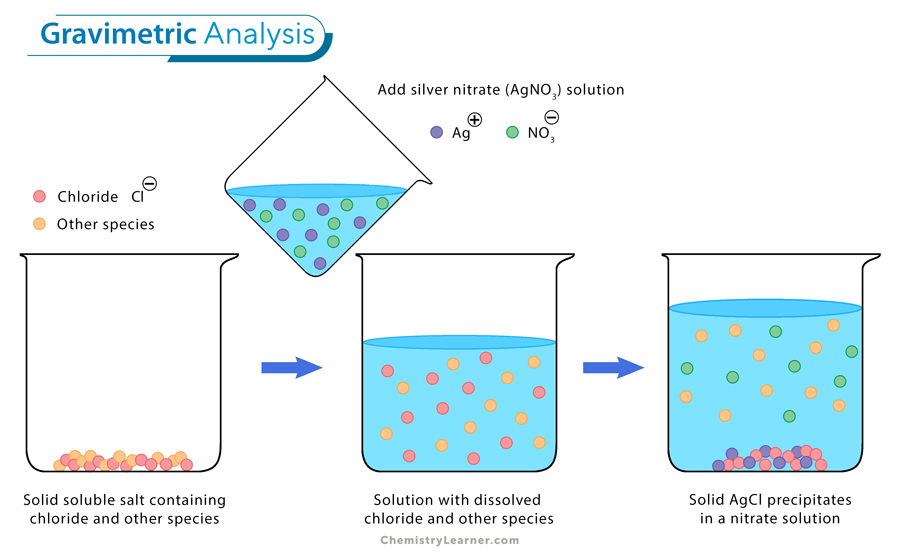
Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate and quantify the analyte of interest. This method is often used in environmental monitoring, pharmaceutical analysis, and quality control in industries such as food and beverage. [1-4]
Principles
Gravimetry is based on the fundamental principles of stoichiometry and mass conservation. The key principle behind gravimetric analysis is that the mass of an isolated compound can be used to calculate the amount of analyte present in the original sample. [4]
Example
One example of gravimetric analysis is determining the chloride content in a sample. In this process, a known volume of the sample solution containing chloride ions is treated with silver nitrate solution to precipitate silver chloride. The formed precipitate is then filtered, dried, and weighed to determine the mass of silver chloride. By knowing the stoichiometry of the reaction and the molar masses involved, one can calculate the amount of chloride ions present in the original sample based on the mass of silver chloride obtained. [4,5]
Types
There are different types of gravimetric analysis techniques, each with its unique approach and applications. Two common types include volatilization gravimetry and precipitation gravimetry. [7]
Volatilization Gravimetry
Volatilization gravimetry relies on separating a volatile component from a sample through heating, which converts it to a gas that is then weighed. This technique is particularly useful for substances that can be easily vaporized under controlled conditions.
Precipitation Gravimetry
Precipitation gravimetry involves forming an insoluble precipitate by adding a specific reagent to the analyte solution. The precipitate is then filtered, dried, and weighed to determine the amount of the analyte in the original sample.
Steps Involved in Conducting a Gravimetric Analysis
Here are the key steps involved in conducting a gravimetric analysis: [3-5]
1. Sample Preparation: The first step in gravimetric analysis is preparing the sample for analysis. This involves selecting an appropriate sample size and ensuring that it is representative of the entire batch.
2. Precipitation: The next step involves precipitating the analyte from the sample solution. It is typically done by adding a reagent that forms an insoluble compound with the analyte, allowing it to be easily separated from the solution.
3. Filtration: Once the precipitate has formed, it needs to be separated from the remaining solution. It is usually achieved through filtration, where the precipitate is collected on filter paper while the filtrate passes through.
4. Washing: Washing the precipitate multiple times removes all traces of impurities.
5. Drying: After washing, the precipitate needs to be dried at a controlled temperature to remove any remaining moisture and ensure accurate weighing.
6. Weighing: The final step in gravimetric analysis involves weighing the dried precipitate using a high-precision analytical balance.
Advantages
Gravimetric analysis is a powerful technique in analytical chemistry that offers several advantages in quantitative analysis. One of the key benefits of gravimetrical analysis is its high precision and accuracy in determining the quantity of a particular substance present in a sample. This method relies on measuring mass, making it reliable for obtaining precise results. [7]
Another advantage of gravimetric analysis is its versatility and applicability to various compounds and elements. This technique can analyze organic and inorganic substances, making it a valuable tool for various industries such as pharmaceuticals, environmental monitoring, and materials science.
Gravimetric analysis also provides valuable insights into a sample’s purity by determining the exact composition of the substance being analyzed. This information is crucial for quality control and compliance with regulatory standards.
This technique allows chemists to obtain precise measurements for research, product development, and process optimization. It is for these reasons that gravimetric analysis is important.
Disadvantages and Limitations
Gravimetrical methods have been a traditional and widely used technique in chemical analysis; however, they come with certain disadvantages and limitations that must be considered. [7]
One of the main challenges faced in conducting a successful gravimetrical analysis is the time-consuming nature of the process. Gravimetric methods often require extended periods for sample preparation, digestion, filtration, and drying, leading to delays in obtaining results compared to more modern analytical techniques.
Another limitation is the sensitivity of gravimetric measurements. The accuracy of these methods can be affected by factors such as environmental conditions, operator errors, and impurities in reagents or samples. It can lower precision and reliability than instrumental methods like spectrophotometry or chromatography.
Additionally, gravimetrical analyses may not be suitable for all samples or analytes. Some compounds may lend poorly to gravimetric determination due to solubility limitations, interference from matrix components, or lack of appropriate precipitation reactions.