Gold
What is Gold
Gold (prounounced as GOLD) is a lustrous and valuable metal belonging to the family of noble metals. Denoted by the chemical symbol Au, it is does not react with other elements or compounds. Pure gold is designated as 24 carats that’s soft in nature, and hence alloyed with other metals. It has 35 isotopes with mass numbers ranging from 171 to 205 of which only Au-197 is stable [1, 2].
Where is Gold Found
It can be naturally found in the free form as well as associated with calcite, quartz, silver, tellurium, lead, zinc, and copper in veins and alluvial deposits. Every year about 1500 tonnes of gold are mined of which two-thirds comes from South Africa and Russia [1, 2].
History
Origin of its Name: It’s derived from the Anglo-Saxon word ‘gold’ and the Sanskrit word ‘jval’ [2].
Who First Discovered Gold: Since the metal is known for more than 5500 years, nobody knows about its original discoverers [1].
When, Where, and How was it Discovered
There are no records of gold’s actual year of discovery since it existed during the prehistoric times in the form of nuggets in river and stream beds. In 2000 BC, the Egyptians actively mined the metal that was obvious from the objects found in the graves of the royal family.
By 640 BC, gold coins made by alloying Au with silver began to circulate in the Kingdom of Lydia. It was only during the reign of King Croesus when pure coins were minted [1].
Identification |
|||
| Atomic number | 79 [1] | ||
| CAS number | 7440-57-5 [1] | ||
| Position in the periodic table [1] | Group | Period | Block |
| 11 | 6 | d | |
Classification, Properties and Characteristics of Gold
General Properties |
||
| Relative atomic mass | 196.967 [1] | |
| Atomic mass/weight | 196.967 atomic mass units [5] | |
| Molar mass/Molecular weight | 196.967 g/mole [6] | |
| Mass Number | 197 | |
Physical Properties |
||
| Color/physical appearance | Golden yellow [1] | |
| Melting point/freezing point | 1064.18°C (1947.52°F) [1] | |
| Boiling point | 2836°C (5137°F) [1] | |
| Density | 19.3 g/cm3 [1] | |
| Standard/Natural state at room temperature (solid/liquid/gas) | Solid [1] | |
| Malleability | Yes [2] | |
| Ductility | Yes [2] | |
| Hardness | 2.5 Mohs [5] | |
| Specific heat capacity | 0.128 J g-1K-1 [4] | |
Chemical Properties |
||
| Flammability | Not flammable | |
| Oxidation state/Oxidation number | −1, +1, +2, +3, +5 [1] | |
Atomic Data of Gold (Element 79)
| Electron configuration (noble gas configuration) | [Xe]4f145d106s1[1] | ||||||
| Atomic structure [5] | |||||||
| – Number of Electrons | 79 | ||||||
| – Number of Neutrons | 118 | ||||||
| – Number of Protons | 79 | ||||||
| Radius of atom | |||||||
| – Atomic Radius | 2.14 Å [1] | ||||||
| – Covalent Radius | 1.30 Å [1] | ||||||
| Ionization energy [1]
(kJmol-1) |
1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| 890.128 | 1949 | – | – | – | – | – | |
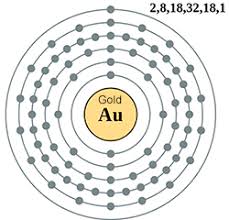
Gold Atomic Structure (Bohr Model)
What are the Common Uses of Gold
- Several types of jewelry are either made from pure gold or alloyed with other metals like copper, silver, palladium, and platinum [1]. The alloys are also used as dental fillings [2].
- Since Au is malleable, it can be beaten into thin sheets called gold leaf and used in gilding to decorate interiors, architecture ornaments, and craft items [2].
- In some countries, coins made of gold are used as a legal tender and monetary exchange medium [1].
- Artificial limb joints, watch gears, junk jewelry, and electrical connectors are plated with the metal to protect against corrosion. A good conductor of electricity as well, it is used in coating electrical copper components [1].
- It is made into thin wires for making circuits in computer chips and motherboards [1].
- Colloidal suspension of Au nanoparticles plays the role of industrial catalysts in large-scale chemical reactions to produce compounds like polyvinyl acetate [1].
Are There Any Toxic Effects of Gold
When administered into body as a part of therapy, excessive accumulation may lead to poisoning, resulting in kidney failure, liver inflammation, platelet dysfunction, nausea, and mouth ulcers [6].
Interesting Facts
- The symbol of the metal originates from the Latin word for gold called aurum [2].
- About 4 grams of Au is present in 1,000,000 tons of water whose recovery cost would exceed the price of the metal itself [1].
- Gold leaf is 400 times thinner than a human hair [2].
Gold Price
The cost of pure (24 carat) gold may vary between $38 and $40 per gram.
- References