Fischer Projection
Definition: What is Fischer Projection?
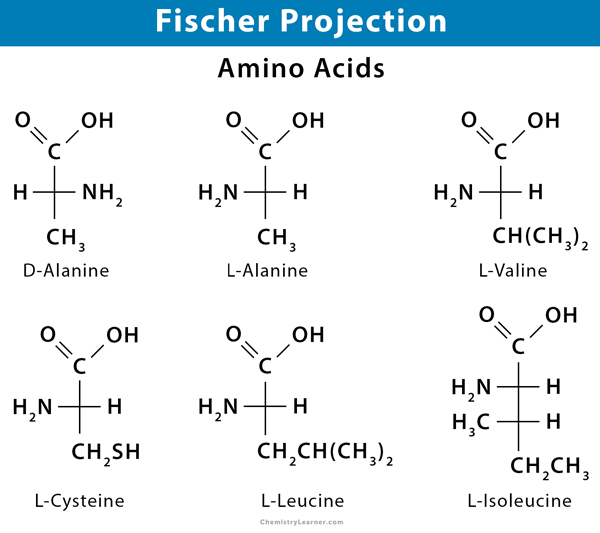
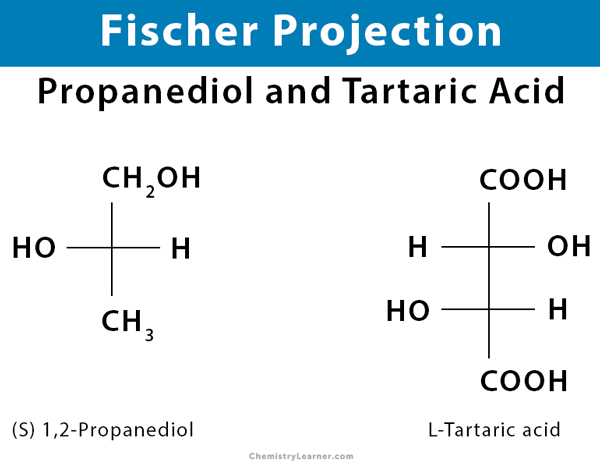
Fischer projections are best used to represent the straight-chain structures of monosaccharides and some amino acids. They represent structural forms that allow one to convey valuable stereochemical information by drawing 3D molecules as flat structures. An advantage of such structures is that they can easily represent multiple stereocenters, and allow easy identification of planes of symmetry.
Fischer projections are useful in depicting monosaccharides (e.g., glucose and fructose) and amino acids (e.g., alanine) because they have many stereocenters or carbons with unique bonds. The different monosaccharides are all quite similar, except that they are different about the orientation of the stereocenters. Crossed lines are used to represent the chiral carbons, hydrogen, hydroxyl, and amino groups.
The Fischer projections were designed by German chemist and Nobel laureate Emil Fischer in 1891.
Types of Sugars
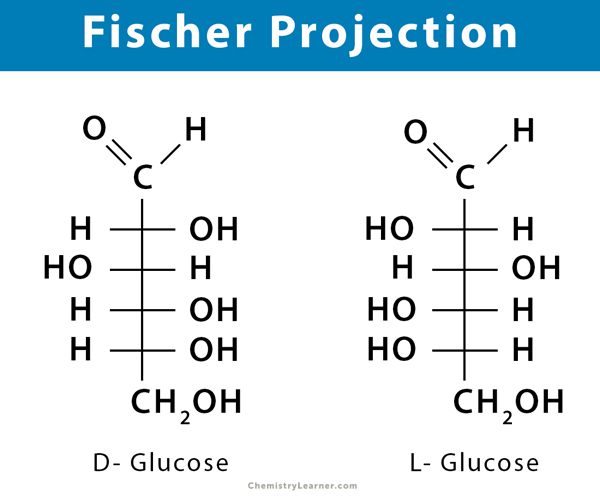
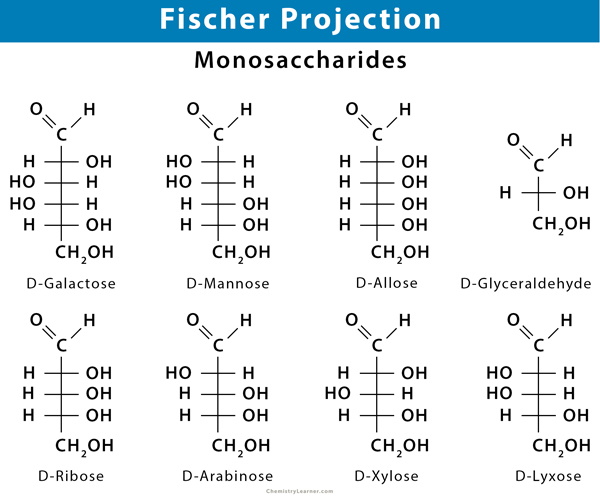
Sugars and amino acids are designated using D- and L- notations. Monosaccharide sugars exist in 4-, 5-, and 6-carbon chains as D- and L-enantiomers. Some common monosaccharides include the following.
Four Carbon Aldehyde or Aldotetrose: Throse and erythrose.
Five Carbon Aldehyde or Aldopentose: Ribose, arabinose, xylose, and lyxose
Six Carbon Aldehyde or Aldohexose: Glucose, galactose, mannose, and allose
Six Carbon Ketone or Ketohexose: Psicose, fructose, sorbose, and tagatose
Besides, there are disaccharides like sucrose, lactose, and maltose. They can be represented by Haworth projection.
How to Distinguish D- and L-Sugars?
- Find the aldehyde functional group at the terminal end of the sugar. This carbon is counted as one. If the sugar is a ketohexose (e.g., fructose), find the ketone functional group and count this carbon as two.
- Number the remaining carbons in chronological order from 2 to 6 for aldohexose and 2 to 5 for aldopentose.
- Find the fifth (for aldohexose) or fourth (for aldopentose) carbon. This carbon is the chiral carbon that is bonded to four different groups.
- If the hydroxyl group on the 5th (4th) carbon is to the right of the molecule, then it is a D-sugar. If the hydroxyl group on the 5th (4th) carbon is to the left of the molecule, then it is L-sugar.
How to Draw Fischer Projection?
In a Fischer projection, the longest chain is drawn vertically. The horizontal lines indicate the bonds with hydrogen, hydroxyl, and amino groups. The four bonds to a chiral carbon make a cross, with the carbon atom at the intersection of the horizontal and vertical lines. The following steps can be employed for an aldohexose.
Step 1: Arrange the molecule so that the chiral carbons and the longest continuous chain are in a vertical line. The aldehyde group representing carbon 1 goes at the top.
Step 2: Draw horizontal lines to make crosses at C-2, C-3, C-4, and C-5.
Step 3: Put the OH groups on the exact side of the cross.
Step 4: Remove C-2, C-3, C-4, and C-5, and the Fischer projection is obtained.
Fischer Projection Rules
The following rules should be kept in mind while working with Fischer projection.
- Fischer projection may be rotated by 180 degrees without changing its meaning.
- Fischer projection may not be rotated by 90 degrees. Such a rotation typically changes the configuration to the enantiomer.
- To find the enantiomer of a molecule drawn as Fischer projection, exchange the right and left horizontal bonds.
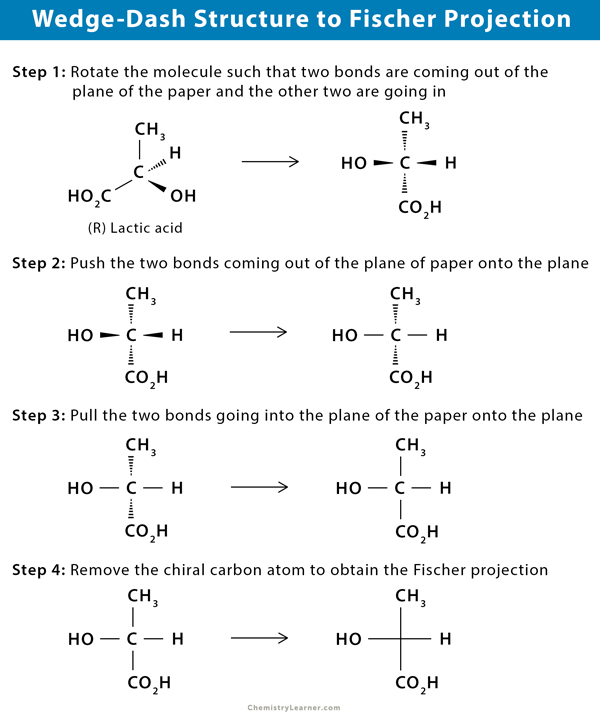
Converting Wedge-Dash Structure to Fischer Projection
The stereochemical formula for (R)-lactic acid can be drawn using the wedge-dashed structure and Fischer projection method. The conversion from wedge-shaped or bond-line structure to Fischer projection is done stepwise.