Finkelstein Reaction
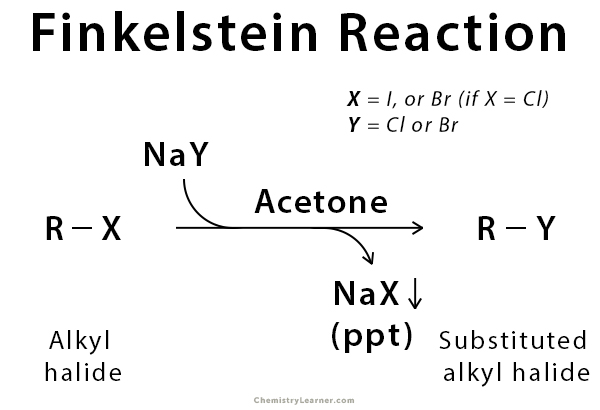
Definition: What is Finkelstein Reaction?
Finkelstein reaction is used in the preparation of alkyl halides using metal halides. It is an SN2 reaction that involves the exchange of one halogen atom for another. In the reaction, newly formed metal halides like sodium chloride and sodium bromide are not soluble in a polar aprotic solvent such as acetone. The reason is that the negative charge on the oxygen atom of acetone is repelled by the negative charge of Cl– and Br– ions. As a result, the insoluble metal halides precipitate and are removed continuously from the solution. Finkelstein reaction is an equilibrium process, and it is driven forward by taking advantage of the poor solubility of these newly formed metal halide salts. Because of the SN2 reaction mechanism, there is an occurrence of inversion of stereochemistry [1-3].
The reaction is named after the German chemist Hans Finkelstein who published his work in a journal in 1910.
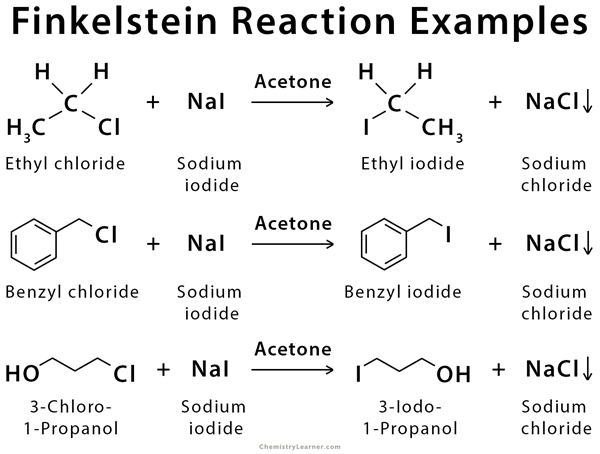
Examples of Finkelstein Reaction
Many new alkyl iodides can be prepared through this method [1,2,4].
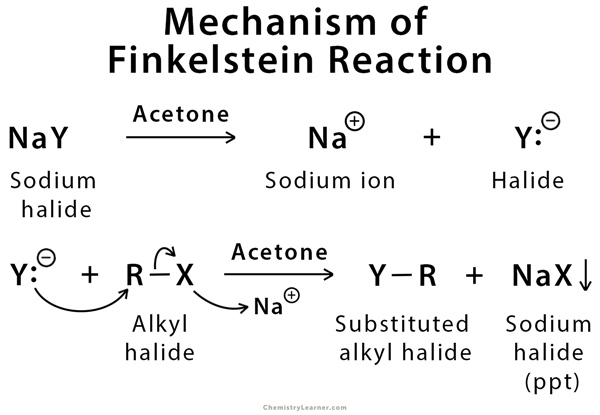
Mechanism of Finkelstein Reaction
It is a simple one-step reaction involving SN2 reaction [1,3,5].
References
- Definition, examples, and mechanism – Chemizi.blogspot.com
- Definition and example – Chem.ucla.edu
- Definition and mechanism – Name-reaction.com
- Example – Cssp.chemspider.com
- Mechanism – Organic-chemistry.org