Europium
What is Europium
Belonging to the family of lanthanides, europium (pronounced as yoo-RO-pee-em) is a rare earth metal denoted by the chemical symbol Eu [1]. It has thirty isotopes with mass numbers ranging from 131 to 162 of which europium-151 and europium-153 are stable, occurring naturally [3].
Where is it Found
As the element does not occur freely in nature, it is found in minerals monazite and bastnasite [1]. It is mined in Australia, USA, India, Russia, and China [6].
History
Origin of its Name: It is named after the continent, Europe [1].
Who Discovered It: It was discovered by the French chemist, Eugène-Anatole Demarçay [1].
When and Where was Europium Discovered
In 1839 after the discovery of cerium, Carl Mosander isolated two new elements, lanthanum, and didymium where the latter contained a mixture of praseodymium and neodymium found later in 1879 by Karl Auer. After this, Paul-Émile Lecoq de Boisbaudran separated another new element, samarium but found it be impure followed by Jean Charles Galissard de Marignac who extracted gadolinium in 1886. Finally, in 1901, Demarçay conducted a series of crystallizations of samarium magnesium nitrate to isolate another new element called europium [1].
Europium Identification |
|||
| Atomic number | 63 [1] | ||
| CAS number | 7440-53-1 [1] | ||
| Position in the periodic table [1] | Group | Period | Block |
| Lanthanides | 6 | f | |
Properties and Characteristics of Europium
General Properties |
||
| Relative atomic mass | 151.964 [1] | |
| Atomic mass | 151.964 atomic mass units [5] | |
Physical Properties |
||
| Color/appearance | Silver [1] | |
| Melting point/freezing point | 822°C (1521°F) [1] | |
| Boiling point | 1529°C (2784°F) [1] | |
| Density | 5.24 g/cm3 [1] | |
| Room temperature at normal phase (solid/liquid/gas) | Solid [1] | |
| Hardness (Vickers) | 3.07 Mohs [5] | |
| Electrical conductivity | 1.6×106 S/m | |
Chemical Properties |
||
| Oxidation state/Oxidation number | +2, +3[1] | |
Atomic Data of Europium (Element 63)
| Valence electrons | 3 [5] | ||||||
| Crystal Structure | Body-centered cube lattice[5] | ||||||
| Quantum numbers [7] | |||||||
| – n | 4 | ||||||
| – ℓ | 3 | ||||||
| – mℓ | 3 | ||||||
| – ms | +1/2 | ||||||
| Electron configuration (noble gas configuration) | [Xe] 4f76s2 [1] | ||||||
| Atomic structure [3] | |||||||
| – Number of Electrons | 63 | ||||||
| – Number of Neutrons | 90 | ||||||
| – Number of Protons | 63 | ||||||
| Radius of atom | |||||||
| – Atomic Radius | 2.35 Å [1] | ||||||
| – Covalent Radius | 1.83 Å [1] | ||||||
| Ionization energy [1]
(kJmol-1) |
1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| 547.109 | 1085.46 | 2404.41 | 4119.9 | – | – | – | |
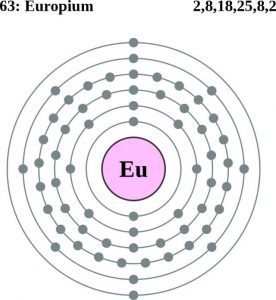
Europium Atomic Structure (Bohr Model)
What are the Uses of Europium
- Europium-doped phosphors are used as fluorescent safety markers on Euro banknotes as they glow red under UV light and therefore help in detecting forgery [1, 2, 8].
- It is used in making low-energy light bulbs to produce soft light like those in incandescent bulbs by maintaining a balance between blue (cold) light and mild red (warm) light [1, 9].
- Europium-doped plastics make good laser materials and super-conducting alloys [1].
Europium Toxicity
The metal may have mild toxic effects, and therefore extreme precautionary measures should be taken while handling it.
Interesting Facts
- The ability of europium to absorb neutrons might be useful in nuclear reactors in the future [2].
- It is the most reactive among all the rare earth metals [2].
How Does Europium Cost
The price of the metal in its pure form may vary between $2300 and $2500 per kilogram.
- References
- http://www.rsc.org/periodic-table/element/63/europium
- https://education.jlab.org/itselemental/ele063.html
- https://pubchem.ncbi.nlm.nih.gov/compound/europium#section=Computed-Properties
- https://www.chemicool.com/elements/europium.html
- http://periodictable.com/Elements/063/data.html
- https://mineralseducationcoalition.org/elements/europium/
- http://chemistry-reference.com/q_elements.asp?Symbol=Eu&language=en
- file:///C:/Users/LENOVO/Downloads/Europium-doped_phosphors_for_lighting_the_past_the.pdf
- https://eic.rsc.org/elements/europium/2020007.article