Ether
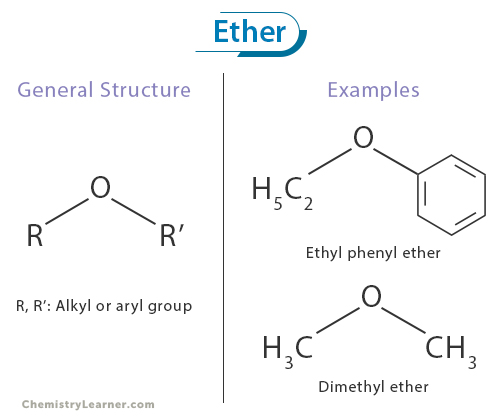
Ether is a broad class of organic compounds characterized by an oxygen atom bonded to two carbon atoms. Ethers can be thought of as oxygen-bridged hydrocarbons with a sweet-smelling and mildly pungent odor. Their defining feature is the R-O-R’ functional group, where R and R’ represent the alkyl or aryl group. [1-4]
Nomenclature [1,2,6,7]
A. Common Naming Conventions
The common names for ethers start with alphabetizing the alkyl or aryl groups bonded to oxygen and adding the word “ether” at the end. For example, the ether with the molecular formula C2H5OC6H5 is commonly known as ethyl phenyl ether.
Ethers featuring oxygen atoms bonded to identical groups on both sides are designated using Greek numerical prefixes like “di” added before the alkyl or aryl group connected to the oxygen atom. For instance, CH3OCH3 is referred to as dimethyl ether.
B. IUPAC Nomenclature Rules
Under the IUPAC system, the parent hydrocarbon is selected based on the substituent group with more carbon atoms. In contrast, the other group connected to the oxygen atom is designated with the prefix “oxy.” For instance, CH3OC2H5 would be denoted as 1-methoxy ethane.
Molecular Formula
Their general molecular formula can be expressed as R-O-R’, where R and R’ represent the alkyl or aryl group. The R groups can be identical or different, giving rise to many ethers with distinct physical and chemical properties. These two carbon-oxygen-carbon linkages distinguish ethers from other oxygen-containing functional groups, such as alcohols or carbonyl compounds. [1-3,6]
Structure
The central oxygen atom is typically sp3-hybridized. The two carbon atoms attached to the oxygen are bonded through single sigma bonds, and two lone pairs of electrons occupy the remaining two positions in the oxygen atom. This spatial arrangement gives ethers a bent or V-shaped geometry with an ideal bond angle of approximately 104.5 degrees, characteristic of tetrahedral structures. The bent shape arises due to lone pairs on oxygen repelling the bonded electron pairs, causing a slight distortion from a perfect tetrahedral geometry. [1-3]
Is Ether Polar
Whether ether is polar is a fundamental consideration in studying organic chemistry. Due to the difference in electronegativity between oxygen and carbon, a polar covalent bond is formed, with oxygen being more electronegative than carbon and thus having a partial negative charge. In contrast, the carbon atoms have a partial positive charge. It results in a dipole moment within the ether molecule. However, the overall polarity of an ether depends on the specific groups (R and R’) attached to the oxygen atom. If the groups on either side of the oxygen atom are identical, the dipole moments cancel each other out, making the molecule nonpolar. If R and R’ are different, the molecule remains polar. [1]
Synthesis
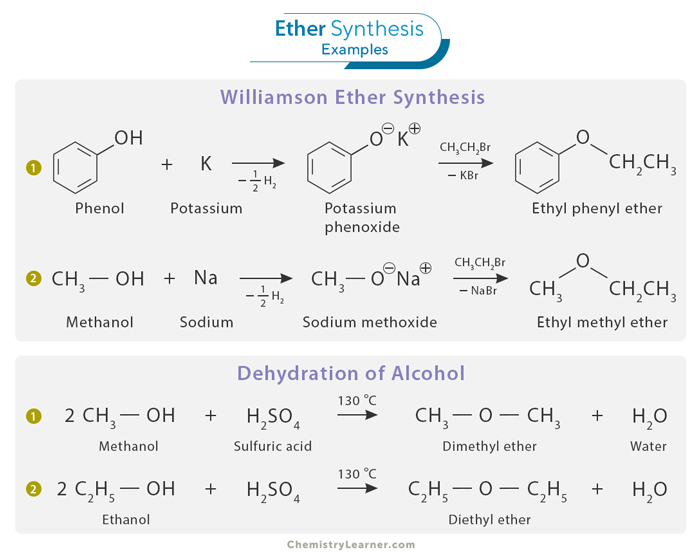
Ethers can be synthesized through several well-established methods, each catering to specific needs and starting materials. Two common approaches to ether synthesis include the Williamson Ether Synthesis and the dehydration of alcohols. [1]
1. Williamson Ether Synthesis
This classic method is widely used to prepare ethers by reacting an alkyl halide with an alkoxide or deprotonated alcohol. The critical step involves the attack of the alkoxide ion on the electrophilic carbon of the alkyl halide. This nucleophilic substitution reaction results in the formation of an ether. Williamson Ether Synthesis is remarkably versatile, allowing for the preparation of symmetrical and unsymmetrical ethers.
2. Dehydration of Alcohol
Ethers can also be synthesized by dehydrating alcohols. An alcohol is treated with a strong acid that facilitates water vapor removal from the alcohol molecule. The resulting product is an ether, with the specific structure determined by the choice of reagents and reaction conditions. The dehydration of alcohols can be a helpful route for synthesizing symmetrical ethers and is commonly employed in laboratory and industrial settings. Only 1o alcohol can be used.
Chemical Reactions
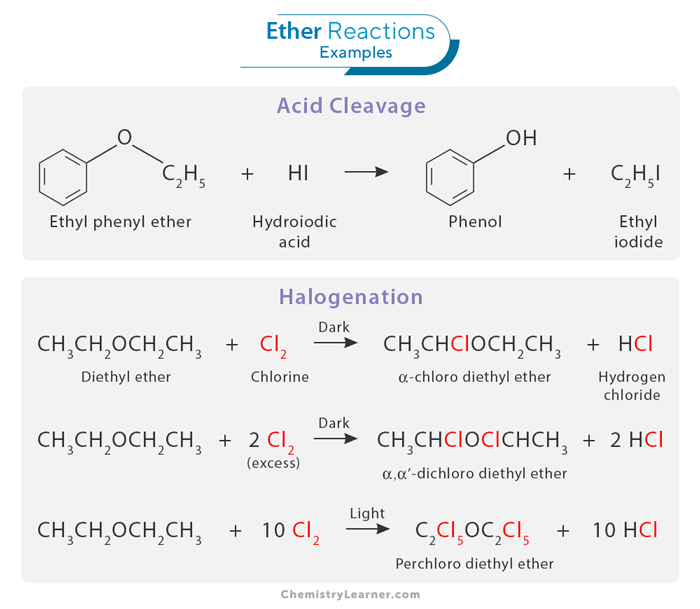
Reactions of ethers encompass a wide range of chemical transformations, reflecting their versatility in organic chemistry. [1,6,8]
1. Cleavage Reactions
Ethers can undergo acidic cleavage when exposed to strong acids. In this process, the oxygen atom of the ether is protonated, creating a highly electrophilic oxonium ion. The resulting positive charge on oxygen makes it susceptible to attack by nucleophiles. This reaction leads to the formation of alcohol and alkyl halide or other electrophilic derivatives, depending on the reagents and reaction conditions.
2. Halogenation
Halogenation involves the introduction of a halogen atom, typically chlorine (Cl) or iodine (I), into the molecular structure of an ether compound. The halogenation of ethers often occurs via a free radical mechanism, where the halogen atom replaces a hydrogen atom attached to the carbon atom adjacent to the ether oxygen. The product of this reaction depends on the reaction conditions. In the dark, the reaction produces an alpha-halogenated ether. However, in the presence of light, all the hydrogen atoms are substituted with the halogen.
3. Combustion
When ignited, ether undergoes a vigorous exothermic reaction, releasing significant heat and producing carbon dioxide (CO2) and water vapor (H2O) as the primary combustion products.
C2H5-O-C2H5 + 6 O2 → 4 CO2 + 5 H2O
Uses
Ethers find various applications across various fields due to their unique chemical properties. [3,5]
- Due to their low polarity and reactivity, ethers are commonly used as laboratory solvents for organic reactions, extractions, and crystallizations.
- Diethyl ether was historically used as a general anesthetic, although safer alternatives have largely replaced it.
- Ethers are also used in formulating various pharmaceutical drugs and as intermediates in synthesizing active pharmaceutical ingredients.
- Modern industrial applications include their use in producing various chemicals, including plastics, rubber, and coatings.





Please email me any information about any books about Ether