Enamine
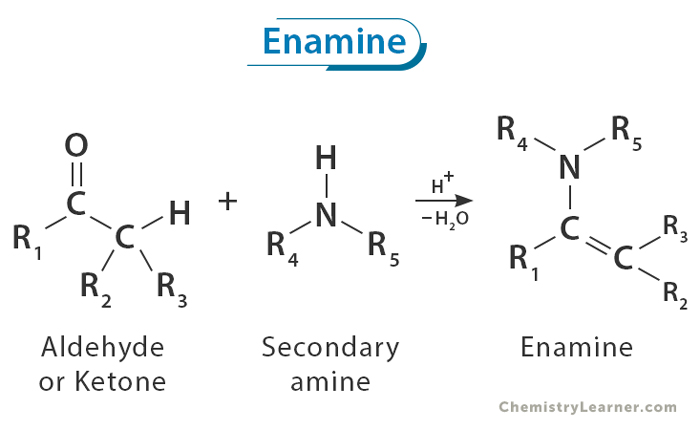
An enamine is a compound that has both an alkene (a molecule that contains a carbon-carbon double bond, C=C) and an amine group (a nitrogen-containing functional group, -NH2). In an enamine, the nitrogen is directly connected to the carbon-carbon double bond. This structure makes enamines reactive and useful for making complex molecules, including pharmaceuticals and other important materials. [1-4]
Enamine Formation
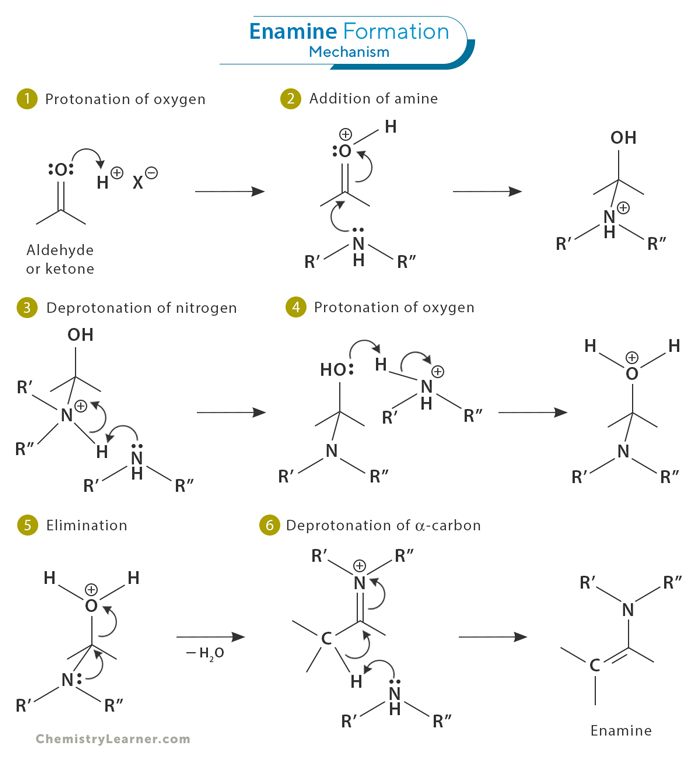
Refer to the mechanism image below.
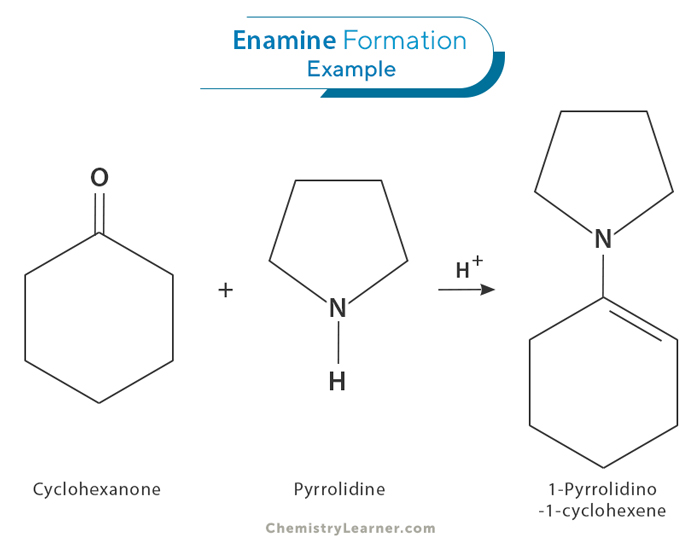
The formation of an enamine is a reversible process that begins when a secondary amine ( where the amino group is directly bonded to two carbon atoms) undergoes nucleophilic addition to the carbonyl group of an aldehyde or ketone (1 and 2). This is followed by a proton transfer, resulting in the formation of a neutral amino alcohol known as carbinolamine (3). Acidic protonation of the oxygen in the carbinolamine enhances its leaving group ability, leading to the elimination of water and the production of an iminium ion (4 and 5). Finally, deprotonation of the α -carbon yields enamine as the final product (6).[1-4]
Properties of Enamine[1-4]
- Structure: It has a unique structure where a nitrogen atom is bonded to a carbon-carbon double bond. This gives the molecule both amine (–NR2) and alkene (C=C) characteristics.
- Reactivity: It can act as a nucleophile in various reactions, participating in Michael additions, alkylations, and Mannich reactions.
- Stability: Compared to a simple imine, an enamine is often more stable due to resonance effects, which lower its reactivity toward hydrolysis.
Enamine Reactions
Enamine’s unique reactivity arises from the electron-rich nature of the nitrogen atom, rendering it proficient in both nucleophilic addition (a type of reaction where a molecule donates electrons to form a new bond) and substitution. [1-4]
1. Alkylation
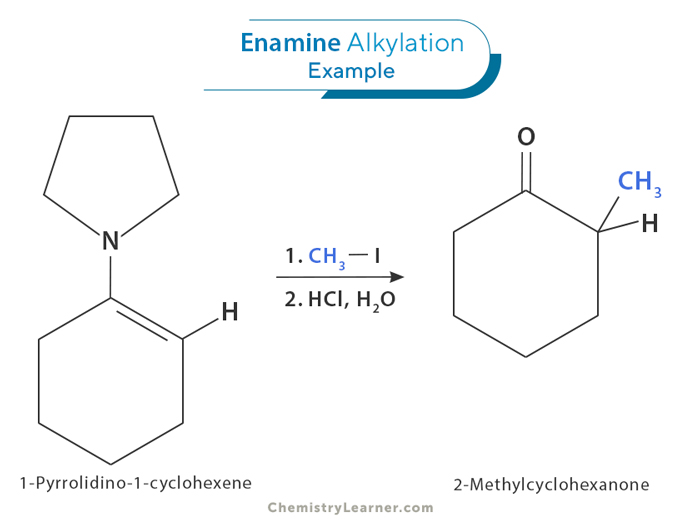
Alkylation of an enamine is a chemical reaction where an alkyl group is added to the nitrogen atom of the enamine. During this process, an alkylating agent reacts with the enamine, forming a new bond between the carbon atoms. This reaction is important for creating more complex molecules in organic chemistry. One of the advantages of alkylation of enamines is that it allows for the selective addition of alkyl groups to specific positions on the molecule, depending on the structure of the enamine. This reaction also occurs under gentle conditions and can be used with many different types of chemical groups.
The alkylation of an enamine has several benefits compared to other methods of forming carbon-carbon bonds. It allows for the selective addition of groups to specific locations on the molecule near the nitrogen atom. This reaction also occurs under gentle conditions and works well with many different types of chemical groups.
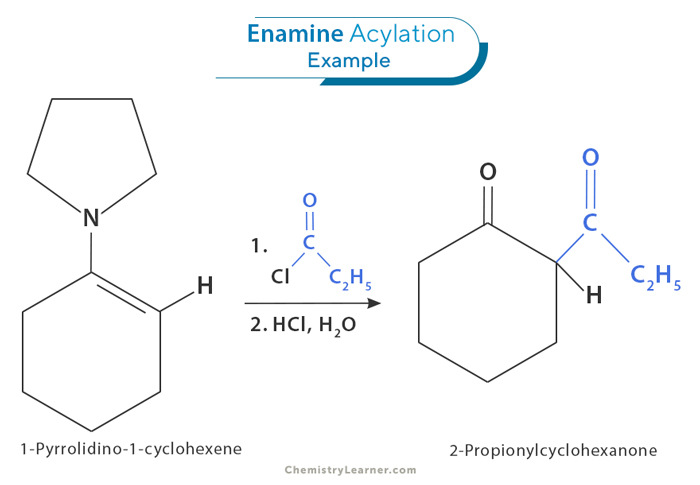
2. Acylation
An acyl is a functional group derived from a carboxylic acid. Acylation is a process where an enamine reacts with compounds like acyl chlorides or acid anhydrides to produce ketones or amides. During this reaction, the nitrogen in the enamine attacks the carbon in the acyl chloride or acid anhydride, leading to the formation of a new product. This reaction usually happens under mild conditions and can be sped up by adding certain chemicals called Lewis acids or bases.
The acylation of enamines is useful in organic synthesis because it makes it easy to create complex ketones and amides from simple starting materials. This reaction also allows for selective changes to enamine molecules, letting chemists add different functional groups to the final product.
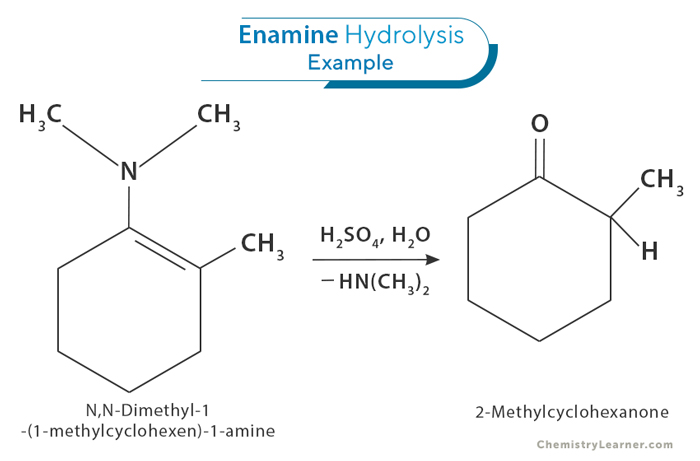
3. Hydrolysis
Hydrolysis is a reaction where a molecule is split into two by the addition of water. In the case of enamines, hydrolysis happens when water reacts with the enamine, breaking the bond between the carbon and nitrogen atoms. This reaction produces an aldehyde or ketone and typically occurs under acidic conditions. An acid, like hydrochloric acid (HCl) or sulfuric acid (H₂SO₄), helps by adding a proton (H⁺) to the nitrogen, making it easier for water to break the carbon-nitrogen bond. After the reaction, you get a carbonyl compound (an aldehyde or ketone) and ammonia or amine as a byproduct, allowing the valuable starting materials to be reused in further synthesis.
Applications [1]
- Versatile Intermediate: Enamines are key intermediates in organic synthesis, enabling the construction of complex molecules with diverse structures and functions.
- Synthetic Reactions: They can undergo various reactions, such as alkylation, acylation, and cyclization, which are essential for creating intricate molecular frameworks.
- Natural Products and Pharmaceuticals: Enamines are valuable in the synthesis of natural products, pharmaceuticals, and biologically active compounds.
- Therapeutic Applications: Enamine-based compounds show potential in treating diseases, including anti-cancer and antimicrobial activities, and as enzyme inhibitors.
- Drug Discovery: Enamines are advantageous in drug discovery due to their ease of synthesis.