Electrolysis
What is Electrolysis
Electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. It uses a direct current (DC) to drive a non-spontaneous reaction that occurs during the process [1-4].
English physicist Michael Faraday popularized electrolysis in the 19th century.
How Does Electrolysis Work
Electrolysis is carried out in an electrolytic cell consisting of a positively charged anode and a negatively charged cathode. For electrolysis to occur, the compound must contain ions. The presence of an electrolyte is essential since it is made up of ionic compounds that have free ions. An external power source like a battery is also required to power the carbon electrodes and drive the cell. The electrical energy is then converted into chemical energy, and the cell does electrical work [1-4].
Ion Interchange
The primary mechanism of electrolysis is the interchange of ions and atoms, resulting in a redox reaction. A chemical change takes place when atoms and ions lose or gain electrons. Positively charged ions or cations move towards the negatively charged cathode. They accept electrons and neutralize. This process is known as reduction, and the cations are said to be reduced.
On the other hand, negatively charged ions move towards the positively charged anode. They lose electrons and get oxidized, a process known as oxidation. The formation of neutral atoms from ions at the electrodes is known as discharging.
Ionic Half Equations
Ionic half-equations represent the process of oxidation and reduction. Suppose M+ is a metal ion that gains electrons (e–) at the cathode to form a neutral atom (M). Then, the half-reaction is given by,
M+ + e– → M
Suppose X– represents a negatively charged nonmetal that loses electrons at the anode and transforms to a neutral atom (X). Then, the half-equation is given by,
X– → X + e–
Predicting Products of Electrolysis
At the electrodes, ions gain or lose electrons, become neutral, and separate from the solution. It is generally easy to predict the products during the electrolysis of molten electrolytes because the compounds split into their elements. The metal part gets deposited as a solid metal. The nonmetal part is released as gas. The electrolysis of aqueous ionic compounds is complicated. Water produces hydrogen ions (H+) and hydroxide ions (OH–) and competes with the primary compound. Hence, hydrogen (H2) and oxygen (O2) gasses can also liberate [5].
Examples of Electrolysis [1-4]
1. Water (H2O)
Water can undergo electrolysis in the presence of an electrolyte like acid or base. The presence of acid improves the electrical conductivity by increasing the hydrogen ion (H+) concentration. Examples of such electrolytes are sulfuric acid (H2SO4) and salt of sodium nitrate (NaNO3). The half-reactions are given below.
At cathode:
2 H+ (aq.) + 2 e– → H2 (g)
Hydrogen gas (H2) will be liberated at the cathode.
At anode:
2 H2O (l) → O2 (g) + 4 H+ (aq.) + 4 e–
Oxygen gas (O2) will be liberated at the anode.
The overall chemical reaction is,
2 H2O (l) → 2 H2 (g) + O2 (g)
For two moles of water, two moles of hydrogen and one mole of oxygen are liberated. The number of moles of hydrogen generated is twice the oxygen. Also, charges are transferred between the electrodes and the electrolyte. For every mole of hydrogen, 2 electrons are transferred from the cathode to the electrolyte. For every mole of oxygen, 4 electrons are transferred from the electrolyte to the anode.
2. Aqueous Sodium Chloride (NaCl)
For electrolysis of aqueous sodium chloride solution, one has to factor in the electrolysis of water. Since water can both oxidize and reduce, it will compete with sodium chloride. However, since sodium is more reactive than hydrogen, hydrogen gas will be released at the cathode.
At cathode:
2 H2O (l) + 2 e– → H2 (g) + 2 OH– (aq.)
At anode:
2 Cl– (aq.) → Cl2 (g) + 2 e–
The overall chemical reaction can be written as,
2 H2O (l) + 2 Cl– (aq.) → H2 (g) + Cl2 (g) + 2 OH– (aq.)
Therefore, the electrolysis of an aqueous sodium chloride solution yields sodium hydroxide, hydrogen, and chlorine.
3. Molten Sodium Chloride (NaCl)
Sodium chloride is melted to its molten state above 800 ˚C before performing electrolysis.
Positively charged sodium ions (Na+) migrate towards the negatively charged cathode and reduce to sodium atoms (Na), forming sodium metal.
Na+ (l) + e– → Na (l)
Negatively charged chloride ions (Cl–) migrate the other way towards the anode and form chlorine gas (Cl2).
2 Cl– (l) → Cl2 (g) + 2e–
The overall chemical reaction is given by,
2 NaCl (l) → 2 Na (s) + Cl2 (g)
In summary, the electrolysis of molten sodium chloride produces metallic sodium and chlorine gas.
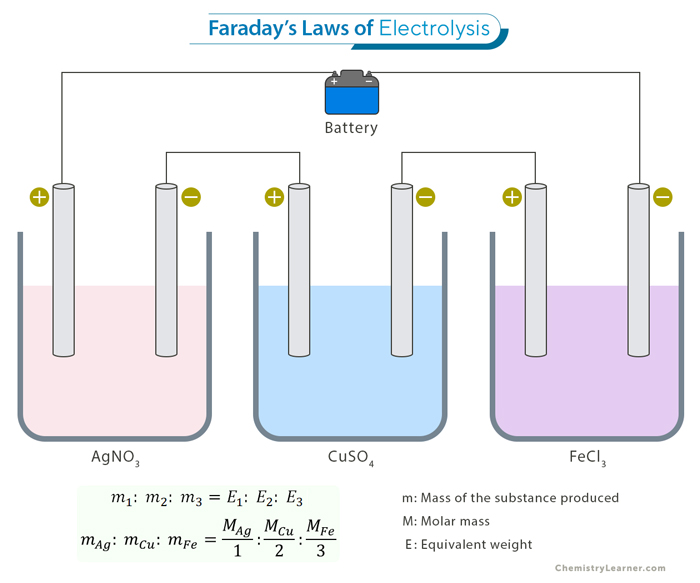
Laws of Electrolysis [1]
During electrolysis, charges transfer between the electrodes and the electrolyte and flow through the electrolyte. The amount of charge flowing per unit time is the current. Based on his experiments, Faraday proposed two laws governing the current and the weight of products formed at the electrodes.
- The weight of products formed during electrolysis is proportional to the current sent through the electrolyte.
- If the current is kept fixed, the weight of the products formed is proportional to each product’s equivalent weight.
The equivalent weight of each product is defined as the molar mass divided by the number of electrons required to neutralize it.
Applications of Electrolysis [6]
1. Extracting, purifying, or cleaning metals: The anode is the impure metal or ore, and the electrolyte is the salt of the metal to be extracted. The pure metal is deposited at the cathode. Aluminum is extracted in this way. Copper can be purified by taking a dilute aqueous solution of copper sulfate and sulfuric acid as the electrolyte.
2. Production of pure chemicals: Many chemicals like sodium hydroxide, caustic soda, potassium permanganate, and potassium chlorate, as well as gasses like oxygen and chlorine, are produced in the industry.
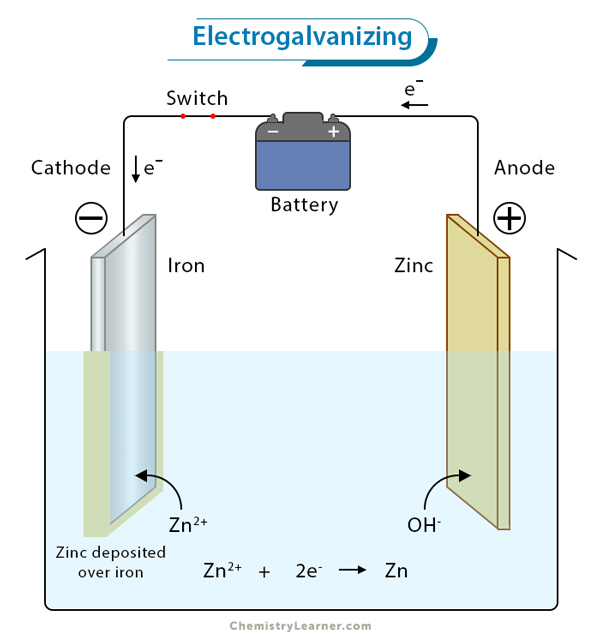
3. Electroplating: It is the process of ornamenting cheap metals like iron with precious metals such nickel, chromium, gold, or silver. It is used for making jewelry, utensils, and auto parts.
4. Electroforming: It reproduces objects like coins and medals by electrodepositing over a mold.
5. Determination of equivalent mass: From the equivalent mass of one metal, one can calculate the equivalent mass of another metal from electrolysis laws.
6. Rust removal: It is used for metal restoration and can remove rust from iron.

Thank you
Perfect
Perfect ????, just what I needed
wow! very understandable
Perfect explanation!
Good. Just what I needed. Thank you.
Thank you for the perfect explanation