Delocalized Electron
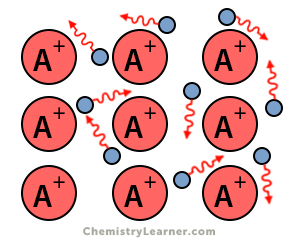
Delocalized electrons are free electrons in a molecule, ion, or solid metal that do not participate in chemical bonding. These electrons are not associated with any atom. Delocalized electrons contribute to the compound’s conductivity. Substances, especially metals, with many delocalized electrons are highly conductive [1-4].
Resonance Delocalization
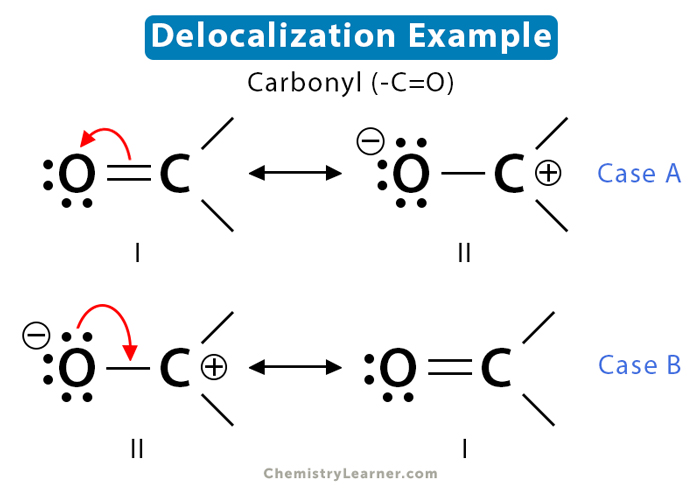
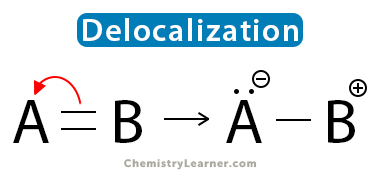
Pi bonds are held by electron pairs loosely attached to the atoms. They form a diffused cloud that can be distorted easily. The pi electrons move around the molecule and become delocalized. One way to visualize delocalization is that electrons flow through the adjacent atom’s orbital. Let us take the example of the carbonyl functional group (-C=O) to understand this concept. The Lewis dot representation is shown below [4].
The C=O bond in carbonyl is polar. The shared electron pair is drawn closer to the more electronegative oxygen atom. The carbonyl group consists of a sigma bond and a pi bond. The pi electrons are dynamically delocalized, as shown below.
The above two structures are resonance structures of the same species. The actual structure is a hybrid of the two.
Formulation of Delocalized Electrons
Curved arrows depict the movement of electrons from one location to another in molecules and ions. Lone pairs also move with relative ease because they are not involved in bonding. The two resonance structures of carbonyl are obtained through delocalization, as shown by case A and case B in the image below. Red curved arrows indicate electron motion. In Case A, the pi electrons move from the pi bond to the more electronegative oxygen. In Case B, a lone pair of electrons on oxygen move toward the positively charged carbon. Therefore, the pi electrons in one structure become a lone pair in another, and vice versa.
How to Identify Delocalized Electrons
Observing its positions in the two resonance structures is the simplest way to tell if electrons are localized or delocalized. If the electrons are located in one position in one structure and another in the other, we can say that delocalization occurs [3].
Delocalization and Stability
Delocalization of electrons stabilizes the system because it spreads energy over a larger area rather than keeping it confined to a small area. Therefore, delocalization brings extra stability to a system compared to a similar system with localized electrons. The stabilizing effect of electron delocalization can be studied from resonance energy [1-3].
Examples
Let us look at a few examples of molecules where delocalization is prominent [1-8].
1. Benzene (C6H6)
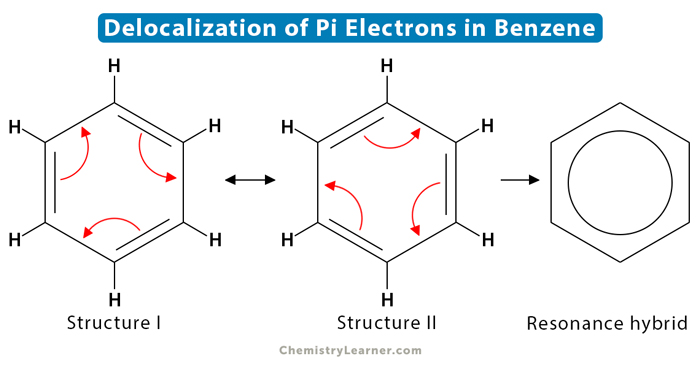
A molecule that contains delocalized electrons is benzene. Benzene has a six-membered ring structure having alternating single and double bonds between the carbon atoms. Such a structure is known conjugated system or conjugated pi bonds. The pi electrons are delocalized over the entire ring. All six carbon atoms equally share these electrons.
Benzene has two resonance structures. Both these structures are equally probable. All C=C bond lengths are the same and equal to 139 pm due to resonance. It makes the molecule extra stable. As with many aromatic structures, the delocalization is shown by a circle in the hybrid resonance structure.
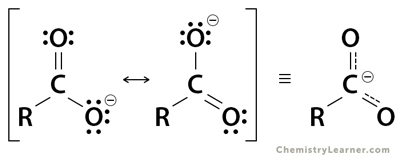
2. Carboxylate (RCOO–)
A carboxylate functional group is the conjugate base of carboxylic acid and has a single negative charge. Two oxygen atoms equally share the negative charge.
3. Metals
Metals have a “sea” of free electrons that move around the atoms. These electrons are not localized to any particular atoms. They carry charges and are responsible for conducting electricity. For this reason, metals are good conductors of electricity.
Localized vs. Delocalized Electrons [3]
- Localized electrons are associated with a particular atom. Delocalized electrons are not associated with any particular atom.
- Localized electrons are found between atoms and are confined to the region between atoms. Delocalized atoms spread across several atoms and are located above and below the atoms, except in graphite.
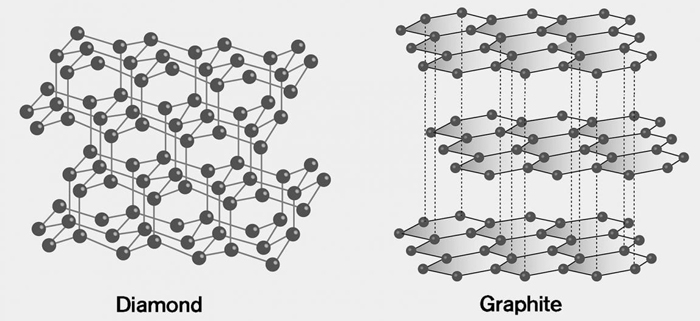
Diamond vs. Graphite
Image Courtesy: Dezeen.com
In the crystal structure of a diamond, four valence electrons participate in bonding, resulting in a tight structure. Hence, the electrons do not roam about freely and are localized. On the other hand, in graphite, three of the four valence electrons are covalently bonded to other carbon atoms. The fourth electron is free to move around the molecule. Since graphite is planar, the delocalized electrons are only confined to a plane. Therefore, graphite conducts electricity along a plane and not perpendicular to it [2].