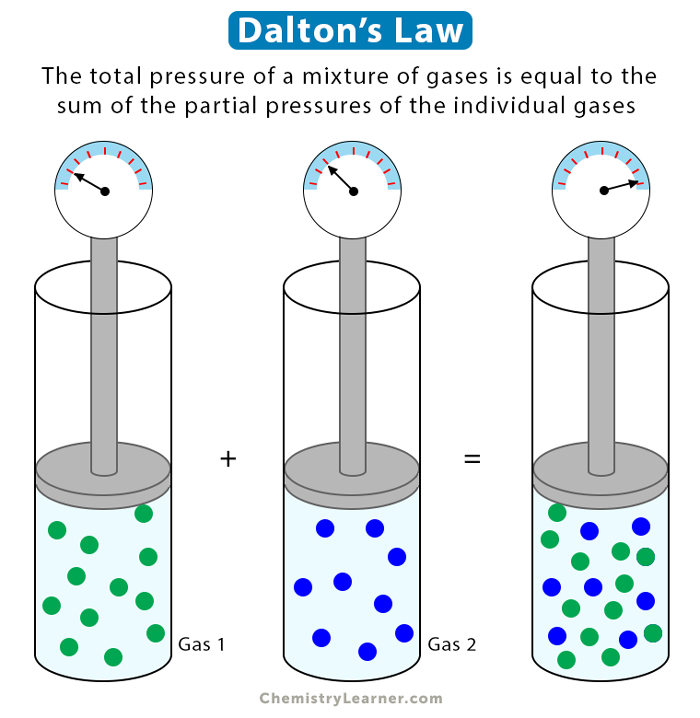
Dalton’s Law
Dalton’s law of partial pressure states that in a mixture of two or more non-reacting gases, the sum of the partial pressures of each gas is equal to the overall pressure of the gas mixture. A gas’s partial pressure is the pressure it would exert on the walls if it were the only gas in a container [1-4].
English physicist and chemist John Dalton published the law in 1802.
Formula
For Dalton’s law to hold good, the following assumptions are made: [1-6]
- The gas molecules occupy an insignificant volume compared to the container’s volume.
- The gases are ideal, meaning there is no attraction among the molecules.
- The pressure is not excessively high. Otherwise, the volume occupied by the molecules becomes significant compared to the free space between them, resulting in a deviation from Dalton’s law.
Based on these assumptions, one can calculate the total pressure of the gas mixture in terms of the partial pressure of the gases. Suppose a mixture contains n gases whose partial pressures are p1, p2, p3, …, pn. Then, the total pressure p of the mixture is given by
p = p1 + p2 + p3 + … + p4
p = Σpi
Partial Pressure from Mole Fraction
Dalton’s law can also be expressed in terms of mole fraction. The mole fraction X is the fraction of the gas present in the mixture. It is evaluated by taking the ratio of the number of moles (ni) of a given gas and the total number of moles (Σni) of all the gases.
Xi = ni /(Σni)
Then, the partial pressure of the i-th gas is
pi = Xi p
Partial Pressure from Ideal Gas Equation
The partial pressure can be calculated from the ideal gas equation. Suppose ni moles of the i-th gas are present in the mixture. The partial pressure is given by
pi = niRT/V
Where
T: Absolute temperature
V: Volume of the gas
Solved Problems
Problem 1: A mixture of oxygen and hydrogen exerts a total pressure of 2.3 atm on the walls of the container. If the partial pressure of hydrogen is 1.2 atm, find the mole fraction of oxygen in the mixture.
Solution:
Given, phydrogen = 1.2 atm p = 2.3 atm
Therefore, the partial pressure of oxygen is
poxygen = p – phydrogen = 2.3 atm – 1.2 atm = 1.1 atm
The mole fraction of oxygen can be found using the following formula.
poxtgen = Xoxygen p
=> Xoxygen = poxygen/p
=> Xoxygen = 1.1 atm/2.3 atm = 0.48
Problem 2: 14 L of oxygen gas at 2.4 atm and 18 L of nitrogen at 2.2 atm are added to a 12 L container at 300 K. Find the partial pressure of oxygen and nitrogen and then find the total pressure.
Solution:
Given Voxygen = 14 L poxygen1 = 2.4 atm Vnitrogen = 18 L pnitrogen1 = 2.2 atm T = 300 K
V = 12 L R = 0.082 L-atm · mol-1 · K-1
The mole fraction of oxygen is
noxygen = (poxygen Voxygen)/(RT) = (2.4 atm · 14 L)/(0.082 L-atm · mol-1 · K-1 · 300 K) = 1.36 mol
nnitrogen = (pnitrogen Vnitrogen)/(RT) = (2.2 atm · 18 L)/(0.082 L-atm · mol-1 · K-1 · 300 K) = 1.61 mol
Therefore, the total number of moles is
n = noxygen + nnitrogen = 1.36 + 1.61 = 2.97 mol
The total pressure is
P = nRT/V = 2.97 mol · 0.082 L-atm · mol-1 · K-1 · 300 K/12 L = 6.09 atm
Therefore, the partial pressures are
poxygen = (noxygen/n) · P = (1.36 mol/2.97 mol) · 6.09 atm = 2.78 atm
pnitrogen = (nnitrogen/n) · P = (1.61 mol/2.97 mol) · 6.09 atm = 3.3 atm