Corrosion
Corrosion is a natural process that occurs when metals and other materials undergo chemical reactions with their environment, resulting in their gradual deterioration. Corrosion, driven by moisture, oxygen, and corrosive substances, hastens metal deterioration, resulting in rust and tarnish. It is essential to understand corrosion as it can have significant economic, safety, and environmental implications. [1-4]
Types of Corrosion
There are several types of corrosion, each characterized by different factors and exhibiting distinct characteristics. Here are some of them: [2-4]
Uniform Corrosion
The first type of corrosion is uniform corrosion, also known as general corrosion. It occurs evenly over the entire surface of the metal, resulting in a gradual thinning or loss of material. This type of corrosion is typically caused by exposure to moisture, oxygen, and other corrosive substances.
Galvanic Corrosion
Another common type is galvanic corrosion, which happens when two dissimilar metals come into contact in the presence of an electrolyte. The more reactive metal acts as an anode and erodes faster than the less reactive metal, acting as a cathode. This type of corrosion can be observed in situations such as plumbing systems or when different metals are used together in structures.
Localized Corrosion
Localized corrosion refers to the attack on specific areas of a metal surface rather than uniformly across it. Pitting corrosion is one example of small pits or holes forming due to localized chemical reactions. Crevice corrosion occurs within narrow gaps or crevices where stagnant electrolytes can accumulate.
Stress Corrosion Cracking (SCC)
Stress corrosion cracking (SCC) occurs when a material experiences the combined effects of tensile stress and a corrosive environment. It can lead to the formation of cracks and, ultimately, the failure of the material. SCC is ubiquitous in metals such as stainless steel and aluminum alloys and can occur in various industries, including aerospace, oil and gas, and nuclear power.
Intergranular Corrosion (IGC)
Intergranular corrosion (IGC) is another form of corrosion that affects materials with distinct grain boundaries. It occurs when these boundaries are susceptible to attack by corrosive agents, leading to localized corrosion along the grain boundaries. This corrosion can weaken the material’s structure and compromise its integrity. Materials prone to IGC include certain stainless steels, nickel-based alloys, and aluminum alloys.
Erosion Corrosion
Erosion corrosion is a complex process involving mechanical erosion and chemical degradation of a material due to exposure to a corrosive environment. It typically occurs when high fluid flow or abrasive particles are present, causing accelerated damage to the material surface. Erosion corrosion can be observed in systems such as pipelines, pumps, turbines, and heat exchangers.
Selective Leaching (Dealloying)
Selective leaching or dealloying refers to the preferential dissolution of one or more components from an alloy when exposed to a corrosive environment. This process often changes the alloy’s composition, resulting in altered mechanical properties and structural weakness. Examples of selective leaching include dezincification in brass fittings used in plumbing systems or desilvering in silver-plated copper contacts.
Examples of Corrosion
Corrosion is a universal natural process that affects various materials, but it is especially pronounced in metals. Here are some illustrative examples of corrosion in metals with chemical reactions: [1,4]
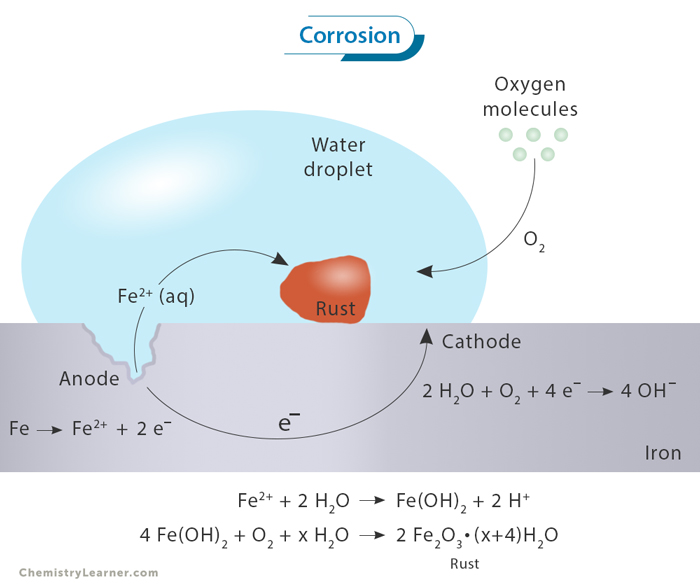
Rusting of Iron
One of the most common and well-known instances of metal corrosion is the rusting of iron. A chemical reaction occurs when iron comes into contact with moisture and oxygen in an electrolyte (usually dissolved salts). Iron undergoes oxidation, leading to the formation of iron oxide, commonly known as rust. The chemical equation for this reaction can be simplified as
4 Fe + 3 O2 + 2 H2O → 2 Fe2O3ˑ2 H2O
The result is a reddish-brown, flaky substance that weakens the structural integrity of the iron, causing deterioration and ultimately compromising its functionality.
Corrosion in Copper
Copper is known for its excellent conductivity and corrosion resistance. However, it is not entirely immune to the effects of corrosive environments. One notable example of copper corrosion is the green patina that forms on copper structures, like the Statue of Liberty. This patina, composed of copper hydroxide and copper carbonate, develops through chemical reactions between copper, moisture, and airborne pollutants. The reactions can be represented as
4 Cu + O2 + 2 H2O + 2 CO2 → 2 Cu(OH)2 + 2 CuCO3
While this greenish layer can act as a protective barrier, preventing further corrosion, it alters the appearance of the metal.
Corrosion of Aluminum
Aluminum is known for its natural resistance to corrosion due to forming a self-protective oxide layer on its surface. However, aluminum can still corrode in specific harsh environments, such as in the presence of chloride ions. The chemical reactions involved include the dissolution of aluminum to form complex ions and the subsequent formation of aluminum hydroxide as the protective layer. The overall process can be described as
4 Al + 3 O2 + 6 H2O + 12 Cl– → 4 Al(OH)3 + 12 Cl–
Prevention of Corrosion
To prevent corrosion, follow these steps: [2,3]
1. Prepare the surface appropriately by removing rust and contaminants before applying protective coatings.
2. Apply coatings like paints, varnishes, or sealants to create a barrier against moisture and corrosive agents.
3. Inspect and maintain materials to promptly identify and address deterioration.
4. Control environmental factors such as humidity, temperature, and exposure to corrosive elements.
5. Use compatible materials to prevent galvanic corrosion when metals with different electrochemical properties might interact.