Conjugation Chemistry
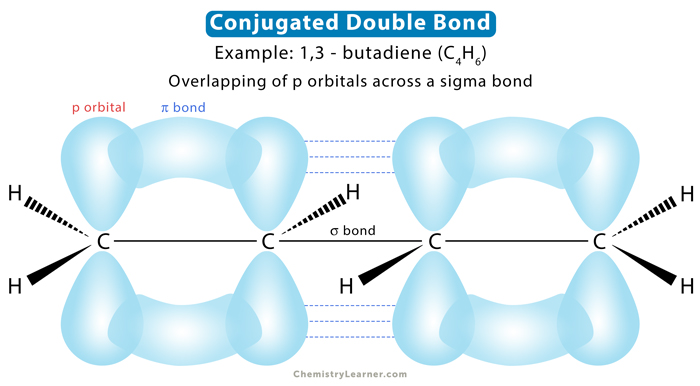
Conjugation refers to the overlapping of p orbitals across sigma bonds. Conjugation occurs under the following two conditions [1-4]:
- The molecule must have at least three p orbitals in a row that overlaps or connect.
- There are delocalized electrons between the overlapping p orbitals.
The conjugate system was first studied by German chemist Johannes Thiel in 1899. Conjugate polymers have a wide range of applications in organic chemistry. Many synthetic compounds, such as polystyrene, polyethylene, and polypropylene, are conjugate systems. Conjugation also occurs in conducting polymers, carbon nanotubules, graphene, and graphite.
Conjugation of P orbitals
The p orbitals are used to determine conjugation. A p orbital includes the following [1-3]:
- An empty p orbital, like a carbocation
- An orbital containing a lone pair, like oxygen, nitrogen, and chlorine
- The p orbital of a pi bond, like another alkene and carbonyl (C=O)
- A half-filled orbital, like a radical
Here are the postulates for conjugation to occur.
- There should be an endless array of p orbitals that can overlap to form pi bonds along the whole system.
- The extended conjugated systems can be acyclic or cyclic compounds. However, they must be flat so that p orbitals can overlap.
- Since the system is planar, the lone pairs will occupy pure p orbital rather than the sp hybrid orbitals, usually found in nonconjugated systems.
- This overlap results in conjugation and allows for the delocalization of pi electrons, a phenomenon known as resonance. It is energetically favorable with increased stability.
- Conjugation of an atom with an adjacent pi bond will alter its electron distribution and bond length. Bond length is inversely proportional to the bond order. The higher the bond order, the shorter the bond length, and vice versa. Therefore, the presence of alpha-hydrogen in the structure increases the bond length.
- If a point in the chain does not possess a p orbital or if (unfavorable) geometry averts the exact alignment, then the conjugation is broken and lost at that point.
Chemical Bonds in Conjugated System [1]
Sigma (σ) Framework System
The bonding scheme is highly localized and pertains to the framework composed of interactions between sp3-, sp2-, and sp-hybridized atomic orbitals on main group elements and 1s atomic orbitals on hydrogen. Additionally, localized lone pairs originating from filled, nonbonding hybrid orbitals are involved.
Pi (π) Framework System
The molecule’s bonding system is formed by unhybridized p-atomic orbital interactions above and below its plane. This interaction is facilitated by sp2- and sp-hybridization on the atoms. The bonding is achieved by adjacent p-orbitals, connected through a link between the atoms, undergoing a side-to-side overlap of their equally sized lobes.
Conjugate Double Bonds
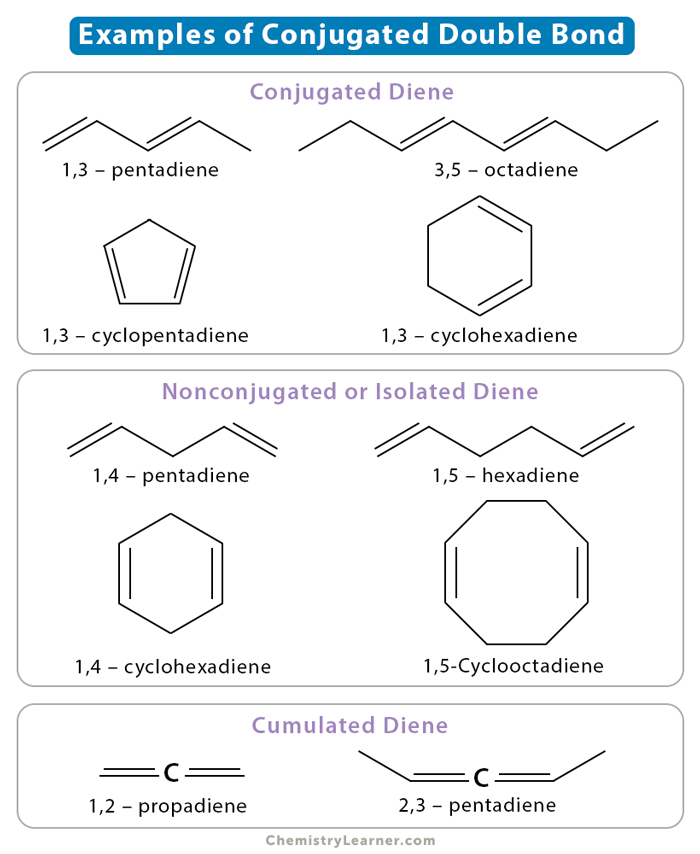
Conjugate double bonds are two carbon-carbon double bonds separated by one or more carbon-carbon single bonds. Such systems are known as dienes. Below are different types of diene systems [1-6].
1. When the double bonds are separated by one single bond, they are known as conjugated dienes, like C=C-C=C. The single and double bonds alternate.
Examples: 1,3 – butadiene; 1,3 – pentadiene; 3,5 – octadiene; 1,3 – cyclopentadiene; and 1,3 – cyclohexadiene
2. When the double bonds are separated by more than one single bond, they are known as isolated or nonconjugated dienes, like C=C-C-C=C. Unlike conjugated dienes, the single and double bonds do not alternate.
Examples: ethene; propene; 1,4 – pentadiene; 1,5 – hexadiene; 2,5 – heptadiene; and 1,4 – cyclohexadiene
3. When the double bonds are adjacent, they are called cumulated dienes, cumulenes, or allenes.
Example: 1,2 – propadiene
Carbon need not be the only atom in conjugation. Other atoms like nitrogen and oxygen can also be in the double bond, like C=C-C=O. Also, conjugated systems are not restricted to only double bonds. Triple bonds can be a part of conjugated systems, like C=C-C≡C.
A significant reaction of conjugated dienes is the Diels-Alder reaction. The diene reacts with a dienophile, resulting in a cyclic olefin.
Stability of Conjugate Dienes
Conjugated dienes are more stable than nonconjugated dienes. The pi-electron density overlap is close and delocalized in conjugated dienes. On the other hand, it spreads across the molecule in nonconjugated dienes. Having more electron density delocalized makes the molecule more stable. Moreover, the positioning of the pi orbitals and the ability for better overlap strengthens the single bond between the two double bonds [2].
The hybridization energy also influences the stability of the structure. The carbon atoms with single bonds in conjugated dienes are sp2 hybridized, and those in nonconjugated dienes are sp3 hybridized. Each orbital has 33% s character in sp2 hybridization and 25% in sp3 hybridization. The conjugate dienes pull the pi electrons, thus making single bonds shorter and stronger than alkenes.
Conjugation vs. Resonance
Conjugation is when three or more p orbitals connect into a more extensive pi system. This conjugated pi system contains pi electrons, which differ from those in the sigma bond. Resonance is the various arrangements of electrons within that pi system and gives rise to different structures [1].