Cerium
What is Cerium
Cerium (pronunciation: SER-ee-em) is a soft, grayish metal that belongs to the group of lanthanides and is represented by the chemical symbol Ce [1, 2]. Its naturally-occurring isotopes include Cerium-136, Cerium-138, Cerium-140, and Cerium-142, out of which Cerium-140 is the most abundant (with 88.48% natural abundance) [3]. Among the 35 known radioisotopes of cerium, the most stable is Cerium-144 that is characterized by a half-life period of 284.893 days [3].
Where is Cerium Found
Cerium is commonly found in rare earth minerals like bastnaesite and monazite [1]. The mineral ore is heated and then treated with hydrochloric acid to produce cerium oxide from which metallic cerium is isolated through electrochemical reduction [1]. The metal can also be obtained through thermal reduction of cerium fluoride with calcium [1, 4]. While China, the CIS countries, and the USA have the highest cerium reserves, the top 3 cerium producing countries in the world are China, Russia, and Malaysia [1].
History
Origin of its Name: It is named after ‘Ceres’, an asteroid that was named after the Roman goddess of fertility and agriculture [1].
Who discovered it: The element was discovered by the Swedish chemists, Jöns Jacob Berzelius along with Wilhelm von Hisinger, and independently by the German chemist Martin Klaproth [1, 4].
When and How was it Discovered
In 1803, Berzelius and Hisinger discovered the new element from a reddish-brown cerium salt called cerite [1, 5]. While investigating the chemical reaction of the cerium salts, they found the two oxidation states – one of which producing yellowish-red salts and the other colorless ones [5]. Although they did not isolate the pure form, they named the element ‘cerium’ and its oxide ‘ceria’ [5].
Martin Klaproth analyzed the salt and concluded that it consists of an oxide of a new metallic element [5]. He named the oxide ‘ockroite’ because of its yellowish-red color [5]. However, the result of Berzelius and Hisinger was published before that of Klaproth, and so the name cerium was approved [5].
Cerium Identification |
|||
| Atomic number | 58 [1] | ||
| CAS number | 7440-45-1 [1] | ||
| Position in the periodic table | Group | Period | Block |
| Lanthanides [1] | 6 [1] | f [1] | |
Properties and Characteristics of Cerium
General Properties |
||||||||||||||||
| Atomic mass | 140.116 amu [1] | |||||||||||||||
| Relative atomic mass | 140.116 [1] | |||||||||||||||
Physical Properties |
||||||||||||||||
| Color | Gray [1, 5] | |||||||||||||||
| Melting point/freezing point | 799 °C, 1470 °F [1] | |||||||||||||||
| Boiling point | 3443 °C, 6229 °F [1] | |||||||||||||||
| Density | 6.77 g cm-3 [1] | |||||||||||||||
| State of matter at room temperature (solid/liquid/gas) | Solid [1, 5] | |||||||||||||||
| Hardness | ||||||||||||||||
| – Brinell | 412 MPa [6] | |||||||||||||||
| – Mohs | 2.5 [6] | |||||||||||||||
| – Vickers | 270 MPa [6] | |||||||||||||||
| Electrical conductivity | 1.4X106 S/m [6] | |||||||||||||||
| Thermal (heat) conductivity | 11 W/(m K) [6] | |||||||||||||||
| Specific heat | 192 J kg-1 K-1 [1] | |||||||||||||||
| Bulk modulus | 21.5 GPa [1] | |||||||||||||||
| Shear modulus | 13.5 GPa [1] | |||||||||||||||
| Young’s modulus | 33.6 GPa [1] | |||||||||||||||
| Vapor pressure | ||||||||||||||||
| – Temperature (K) | 400 | 600 | 800 | 1000 | 1200 | 1400 | 1600 | 1800 | 2000 | 2200 | 2400 | |||||
| – Pressure (Pa) | – | – | – | 2.47 X 10-11 | 8.91 X 10-8 | 2.97 X 10-5 | 2.33 X 10-3 | 6.91 X 10-2 | 1.04 | 9.56 | 60.8 | |||||
Chemical Properties |
||||||||||||||||
| Oxidation state/Oxidation number | +2,+3, +4 [1] | |||||||||||||||
| Isotopes | Isotope | Mass | Abundance (%) | Half-life | Mode of decay | |||||||||||
| 136Ce | 135.907 | 0.185 | >0.7 X 1014 y | EC EC | ||||||||||||
| >4.2 X 1015 y | β-β- | |||||||||||||||
| 138Ce | 137.906 | 0.251 | >3.7 X 1014 y | EC EC | ||||||||||||
| 140Ce | 139.905 | 88.45 | – | – | ||||||||||||
| 142Ce | 141.909 | 11.114 | >1.6 X 1017 y | β-β- | ||||||||||||
Atomic Data of Cerium (Element 58)
| Valence electrons | 2 [7] | |||||||
| Quantum numbers | ||||||||
| – n | 4 [7] | |||||||
| – ℓ | 3 [7] | |||||||
| – mℓ | -2 [7] | |||||||
| – ms | +1/2 [7] | |||||||
| Electron configuration (noble gas configuration) | [Xe] 4f15d16s2 [1] | |||||||
| Atomic structure | ||||||||
| – Number of electrons | 58 [5] | |||||||
| – Number of neutrons | 82 [5] | |||||||
| – Number of protons | 58 [5] | |||||||
| Radius of Atom | ||||||||
| – Atomic radius | 2.42 Å [1] | |||||||
| – Covalent radius | 1.84 Å [1] | |||||||
| Electronegativity (Pauling-scale) | 1.12 [1] | |||||||
| Electron affinity | 62.72 kJ mol-1 [1] | |||||||
| Ionization energy (kJ mol-1) | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th |
| 534.403 | 1046.87 | 2697.73 | 3546.608 | 6324.61 | 7487.3 | – | – | |
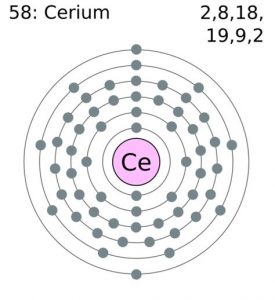
Cerium Electron Configuration (Bohr Model)
What is It Used for
- The mischmetal alloy made of cerium, lanthanum, neodymium, and praseodymium mixed with magnesium oxide and iron oxide is used as flints for lighting cigarettes and gas [1, 5].
- Cerium oxide, as a catalyst, is used in self-cleaning ovens for preventing the accumulation of cooking residues and for polishing glass surfaces [1, 5].
- It is used in catalytic converters because it helps reduce automobile exhaust emissions [1]. Cerium oxide nanoparticles are added to diesel for reducing the emission of soot and improving engine performance of vehicles [5].
- Cerium sulfide is non-toxic by nature and is used as a coloring pigment because of its rich red color [1].
- The element is used in phosphors in flat-screen televisions, floodlights, and energy-efficient light bulbs [1].
- Flammacerium, a compound containing cerium nitrate and silver sulfadiazine, is a topical ointment used for treating infections in burn wounds [5].
Cerium Toxicity
The element 58 has no known biological roles and is low to moderate in toxicity [1, 5].
Interesting Facts
- The image of cerium is a graphical representation of the asteroid Ceres, whereas the background is derived from an astronomical map of the 17th-century [1].
- Its natural abundance is almost similar to zinc, but it is more than lead or tin [1].
- At room temperature, the element can readily oxidize [4].
- Pure cerium may ignite if scratched with a sharp metal object such as a knife [4].
- It decomposes in water to produce cerium hydroxide and hydrogen [5].
Cost of Cerium
The cost of 100 grams of pure cerium is about $380, but in bulk, it is about $1.20 [5].
- References
- http://www.rsc.org/periodic-table/element/58/cerium
- https://education.jlab.org/itselemental/ele058.html
- https://www.webelements.com/cerium/isotopes.html
- https://www.livescience.com/37606-cerium.html
- https://www.chemicool.com/elements/cerium.html
- http://periodictable.com/Elements/058/data.html
- http://chemistry-reference.com/q_elements.asp?Symbol=Ce&language=en








