Catalytic Reaction (Catalysis)
What is a Catalytic Reaction [2,4-6]
Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any change.
Characteristics of Catalytic Reaction [6]
- The catalyst remains unchanged in mass and chemical composition at the end of the reaction.
- Only a small quantity of catalyst is generally needed.
- A catalyst cannot initiate a reaction. The function of a catalyst is only to alter the speed of the reaction, already occurring at a particular rate.
- A catalyst does not alter the position of equilibrium in a reversible reaction.
- The catalyst is generally specific in its action.
- Selectivity of catalyst is the ability of a catalyst to direct a reaction to yield a particular product. It means that different products are obtained when different catalysts are used in reactions between the same reactants. For example,
CO+3H2 → CH4+H2O (when Ni is used as a catalyst)
CO+H2 → HCO (when Cu is used as a catalyst)
Types of Catalytic Reaction [1-3,5]
1. Homogenous Catalysis
In this type of catalysis reaction, both catalyst and reactant remain in the same phase. Here, the catalyst either remains uniformly dispersed in the gas phase or gets dissolved in the reactant. So, the number of collisions between reactant and catalyst is maximum. These catalysts are used in various industrial applications. They allow for an increase in reaction rate without an increase in temperature.
Now, let us understand the process of homogeneous catalysis by citing the example of the decomposition of ozone.
As we know, ozone is formed when oxygen molecules absorb ultraviolet light.
3O2 → 2O3
Ozone is a relatively unstable molecule that decomposes to yield diatomic oxygen.
O3 → O2 + O
Nitric oxide (NO) acts as a catalyst in the decomposition of ozone. The presence of NO influences the rate of this decomposition reaction.
NO + O3 → NO2 + O2
NO2 + O3 → NO + 2O2
The nitric oxide reacts and is regenerated after ozone is decomposed. As it is not consumed in the reaction, it acts as a catalyst. Therefore, the decomposition rate of ozone is higher in the presence of nitric oxide because of the catalytic activity of NO.
Example: Hydrolysis of an ester like ethyl acetate (CH3COOC2H5) in the presence of an acid catalyst (aq. H+) yields acetic acid (CH3COOH) and ethyl alcohol (C2H5OH).
CH3COOC2H5 + H2O → CH3COOH + C2H5OH
2. Heterogeneous Catalysis
Unlike homogenous catalysis, the catalyst exists in a different phase than the reactant in this type of catalysis reaction. Generally, the catalyst remains solid, while the reactants are gaseous or liquid. The catalyst does not dissolve into the reacting mixture. Instead, the catalytic action occurs by adsorption, where catalyst acts as adsorbent and reactant acts as adsorbate.
The whole process occurs in four steps:
1. During the process, the reactant molecules get attached or adsorbed on the catalyst’s surface by chemical bonding.
2. The adsorbed reactants get activated.
3. Then, the adsorbed reactants get converted into products on the surface of the catalyst.
4. After the formation of the product, the product molecules leave the surface of the catalyst. This diffusion of the product from the surface into the gas or liquid phase is called desorption.
Example:
i) Preparation of SO3 by contact process in the presence of catalyst V2O5 (Vanadium pentoxide)
2SO2 + O2 → 2SO3
ii) Preparation of NH3 by Haber’s process using iron (Fe) catalyst
N2 + 3H2 → 2NH3
3. Enzyme Catalysis
Enzyme catalysis is a process in which the reaction rate is altered in the presence of a biomolecule, enzyme. An enzyme is a protein molecule, which acts upon a particular substrate only. It bears an active site on which the substrate molecules attach and convert into products. Upon conversion, the product molecules detach themselves from the enzyme. So, in the end, the enzyme molecule remains unchanged.
The fundamental mechanism of enzyme catalysis follows the induced-fit model. First, the enzyme (E) binds with its specific substrate (S) molecule, forming the enzyme-substrate complex (E-S complex). Then, the substrate gets converted within the E-S complex. Finally, the products (P) get dissociated from the complex, leaving the enzyme unchanged.
Step I: E + S → E-S complex
Step ii: E-S complex → E + P
Example:
i) Decomposition of hydrogen peroxide (H2O2) into oxygen (O2) and water (H2O) in the presence of catalase.
H2O2 → O2 + H2O
ii) Decomposition of sucrose (C12H22O11) into glucose (C6H12O6) and fructose (C6H12O6) in the presence of sucrase.
C12H22O11 → C6H12O6 + C6H12O6
4. Acid-base Catalysis
In acid-base catalysis, the rate of a chemical reaction is altered by adding an acid or a base. However, the acid or base itself is not consumed in the reaction. Acid and base are not required together for every reaction. Some reactions are acid-specific, known as acid catalysis. The base-specific ones are called base catalysis.
The mechanism of this reaction can be explained in terms of the Brønsted-Lowry theory. According to this theory, protons can transfer from an acidic catalyst to the reactant or from the reactant to a base catalyst. Electrons can also transfer from the base catalyst to the reactant or from the reactant to an acid catalyst.
Examples
i) Decomposition of sucrose into glucose and fructose in the presence of sulfuric acid (H2SO4)
C12H22O11 → C6H12O6 + C6H12O6
ii) Addition of hydrogen cyanide (HCN) to aldehydes and ketones. Propanone (CH3CCH3=O) reacts with HCN in the presence of sodium hydroxide (NaOH) to give 2-hydroxy-2-methylpropanenitrile ((CH3)(CH3)C(OH)(CN)).
CH3CCH3=O + HCN → (CH3)(CH3)C(OH)(CN)
Catalysis and Reaction Kinetics [5-6]
From the above discussion, catalysts have a significant role in reaction kinetics. They alter the rate of a chemical reaction by providing an alternative pathway. However, all the catalysts do not act in the same way. Most of them speed up the chemical process, while some impede it.
The ones which accelerate the rate of a chemical reaction is referred to as positive catalyst. On the other hand, the catalysts that decelerate the chemical reaction rate are known as negative catalysts or inhibitors.
For instance, oxygen is an inhibitor of free-radical reactions. Such reactions must be performed in an oxygen-free environment, e.g., under a blanket of nitrogen.
Another interesting phenomenon, called autocatalysis or self-catalysis, may also occur. Here, one of the products of the reaction acts as a catalyst for that reaction. For example, in the reaction of permanganate ion (MNO4-1) with oxalic acid (C2H2O4) in acidic solution (H+), water (H2O), carbon dioxide (CO2), and manganous ion (Mn+2) are formed. Among them, the manganous ion acts as an autocatalyst. These reactions are potentially dangerous since the reaction rate may increase to the point of explosion.
2MnO4-1 + 5C2H2O4 + 6H+ → 2Mn+2+ 8H2O +10CO2
Some substances increase the efficiency of catalysts, but they are not catalysts. Such substances are called promoters. Molybdenum (Mo) acts as a promoter in Haber’s process of producing ammonia. It enhances the efficiency of the iron catalyst.
Substances that react with catalysts to reduce or eliminate their effect are called poisons. For instance, hydrogen sulfide (H2S) or carbon monoxide (CO) destroys the activity of the iron catalyst in Haber’s process.
N2 + 3H2 → 2NH3
[Catalyst – Fe, Promoter – Mo, Poison – H2S or CO]
Mechanism of Catalytic Reaction [5-6]
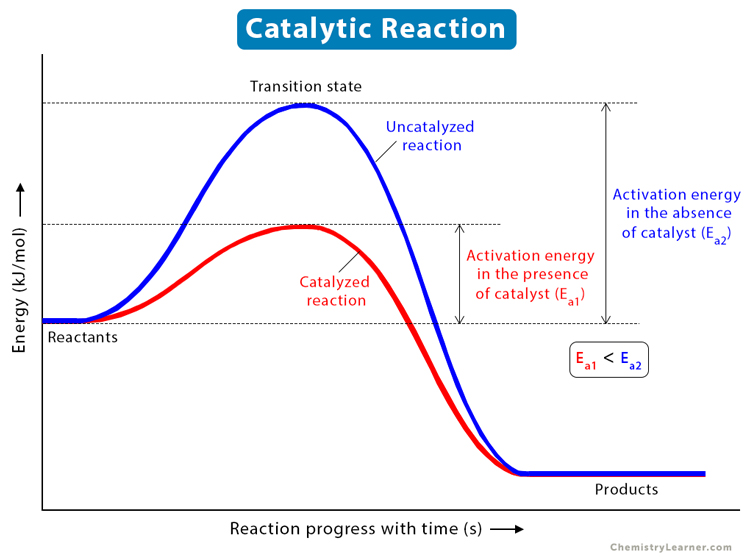
Catalysts lower the activation energy (Ea) of the reaction’s transition state by providing an alternative pathway for the reaction. Activation energy is the minimum energy needed for the reaction to occur. Catalysts do so by providing an alternative pathway for the reaction. They orient the reacting particles in such a way that more successful collisions occur among them. They also form an intermediate or activated complex by reacting with the reactants. The formation of the activated complex requires lower energy to form the product.
The mechanism of the catalytic reaction is as follows:
1. A+C → AC
2. B + AC → ACB*
3. ACB* → C + D
[A and B are reactants, C is the catalyst, D is product]
Here, ACB* is the intermediate or activated complex. Although the catalyst takes part in the first step, in the end, it is rereleased. The catalyst has provided an alternative set of reaction steps, which we refer to as an alternative pathway. The catalyst pathway requires less activation energy and is, therefore, faster, unlike the uncatalyzed reaction.
Catalyzed vs. Uncatalyzed Reactions [6]
The uncatalyzed or non-catalytic reaction is the reaction that does not occur under the influence of a catalyst.
Example: Thermal decomposition of calcium carbonate (CaCO3) in calcium oxide (CaO) and carbon dioxide (CO2).
CaCO3 + Heat → CaO + CO2
Difference between catalyzed and uncatalyzed reaction
| Catalyzed reaction | Uncatalyzed reaction |
|---|---|
| Requires less activation energy | Requires more activation energy |
| Two-step mechanism | One-step mechanism |
| Two transition states are observed | One transition state is observed |
| Catalyst alters the rate of reaction | No external influence alters the rate of reaction |
FAQs
Ans. Catalytic cracking is the process through which complex hydrocarbons get broken down into simpler molecules.
Ans. Catalytic converters use reduction and oxidation (redox) reactions to reduce harmful emissions. They use a reduction catalyst composed of platinum and rhodium that helps to reduce nitrogen oxides (NOx) by removing nitrogen and oxygen atoms from nitrogen oxide molecules (NO and NO2). The free oxygen forms oxygen gas (O2).
NOx → Nx + Ox
Ans. Industrial ethanol (CH3CH2OH) is produced by a catalytic reaction of ethylene (CH2═CH2) with water at high pressures and temperatures.
References
- Catalysis – Courses.lumenlearning.com
- Catalytic reactions – Chem.libretexts.org
- Catalysis – Opentextbc.ca
- Catalysts – Energy.gov
- Kinetics of Catalysis – Chem.libretexts.org
- Catalysis – Openstax.org