Carbanion
A carbanion is an anion with a negatively-charged carbon atom. The negative charge is due to a lone pair of electrons in the outermost shell of the carbon atom. A carbanion has a high concentration of electron density in the negatively-charged carbon and efficiently attracts many electrophiles. It forms a reactive intermediate in many organic reactions.
Carbanions are encountered in organic chemistry in elimination reactions. Also, they are found in organometallic chemistry, especially Grignard reaction and alkyl lithium chemistry.
Example
A carbanion is a conjugate base (R3C–) of a carbon acid (R3CH). Any molecule containing a C-H bond can lose a proton and form a carbanion.
R3C-H + B– → R3C– + HB
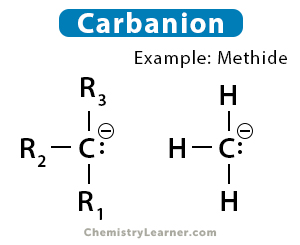
The simplest example of a carbanion is methide ion (CH3–). It is derived from methane by removing a proton.
CH4 ⇋ CH3– + H+
Other carbanions include benzylic carbanion (C6H5CH2–).
Structure
The negatively-charged carbon has three substituents, making the number of valence electrons eight. Without pi-electron delocalization, the lone pair remains in a spx hybridized orbital. As a result, carbanions assume trigonal pyramidal, bent, and linear geometries. If the lone pair is in an orbital with a high percentage of s character, pyramidal and bent geometries are favored. This phenomenon is opposite to carbocation, where unoccupied orbitals have a p character resulting in planar and linear geometries.
Delocalized carbanions deviate from these geometries. In such structures, the lone pair occupies a pure p orbital or one with a high p character. p orbitals conjugate efficiently, resulting in an effective charge delocalization. As a result, alkyl carbanions with neighboring conjugate groups, like allylic anions, have planar geometries. Similarly, delocalized alkenyl carbocations acquire linear geometries.
Stability
Several factors affect the stability of the carbanion molecule.
1. Induction
If the substituent group is highly electronegative, it draws the shared electrons towards itself and stabilizes the molecule. On the other hand, if the substituent group is electropositive, it will “push” the shared electrons toward the carbanion, making the molecule less stable. There more the substitution, the more stable is the molecule.
2. Hybridization
The hybridization of the negatively-charged carbon is also another factor determining stability. The greater the s character, the more stable the anion.
3. Resonance
Conjugation is another factor that affects stability. Delocalization distributes the negative charge all over the molecule, resulting in resonance. Aromatic systems offer tremendous stability through this resonance effect when they are present as a substituent.





Thanks