Brønsted-Lowry Theory
What is the Brønsted-Lowry Theory?
The Brønsted–Lowry theory describes the interaction between an acid and a base in terms of proton transfer. According to this theory, a substance can function as an acid only in the presence of a base. Similarly, a substance can function as a base only in the presence of an acid. All acid-base reactions involve the transfer of an H+ ion or proton. The Brønsted-Lowry theory states that any compound that can transfer a proton to any other compound is an acid, and the compound that accepts the proton is a base. This theory is a generalization of the Arrhenius theory.
The history of this theory goes back to 1923 when a Danish chemist Johannes Nicolaus Brønsted and an English chemist Thomas Martin Lowry independently developed it.
Brønsted-Lowry Acids and Bases
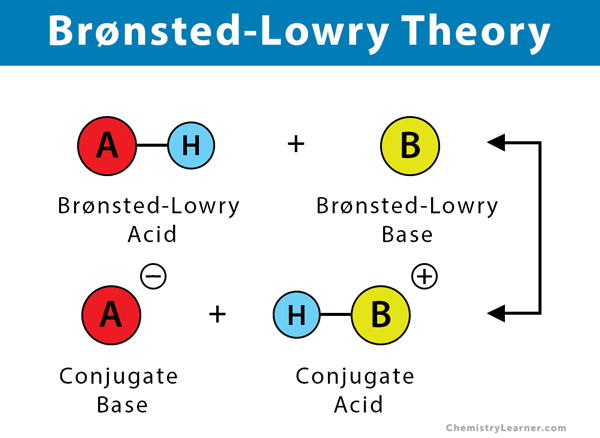
A Brønsted-Lowry acid must contain at least one hydrogen atom, typically attached to a very electronegative atom such as oxygen. When a Brønsted-Lowry acid donates a proton, it loses one H from its molecular formula, and its charge decreases by one. The resulting species is a base since the reversal of the loss of a proton is the gain of a proton. The species gaining the proton is acting as a proton acceptor or a Brønsted-Lowry base.
Furthermore, when an acidic substance loses a proton, it forms a base, called the conjugate base of the acid. When a basic substance gains a proton, it forms an acid called the conjugate acid of a base.
Examples of Brønsted-Lowry Acids and Bases
Some examples of Brønsted–Lowry acids are ammonium (NH4+), hydronium (H+), and hydrated metal cation [Al(H2O)6]3+.
Some examples of Brønsted–Lowry bases are acetate (CH3COO–), phosphate [(PO4)3-], carbonate (CO32-), sulfide (S2-), and halide (X–).
Because of its ability to both accept and donate protons, water is known as an amphoteric or amphiprotic substance. This capability means that water can act as either a Brønsted-Lowry acid or a Brønsted-Lowry base.
Strong and Weak Brønsted-Lowry Acids and Bases
The strength of a base is measured by its ability to capture protons. In contrast, the strength of an acid is measured by its ability to donate protons. Therefore, the stronger the acid, the weaker the conjugate base, and the stronger the base, the weaker its conjugate acid.
Brønsted-Lowry Acid-Base Reaction
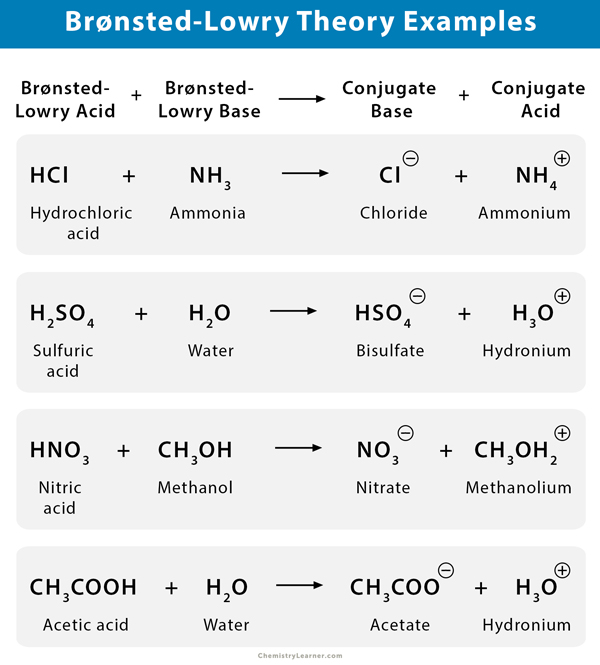
According to the Brønsted-Lowry theory, an acid-base reaction involves the transfer of a proton from the acid to the base. The following reaction defines this concept.
Acid + Base ⇌ Conjugate Base + Conjugate Acid
Consider the example of hydrochloric acid (HCl) reacting with base ammonia (NH3).
HCl + NH3 → NH4+ + Cl–
In this reaction, HCl donates its proton to NH3. Therefore, HCl is a Brønsted-Lowry acid. Since NH3 has a lone pair that it uses to accept a proton, it is a Brønsted-Lowry base.
Advantages of Brønsted-Lowry Theory
- It explains the behavior of acids and bases in both aqueous and non-aqueous solvents.
- It can explain the basic character of substances like Na2CO3, which do not contain an OH group. Hence, it is not a base according to Arrhenius’ concept on the basis that it cannot accept a proton.
- It is not limited to molecules but also covers even the ionic species to act as acids or bases.
Limitations and Drawbacks of Brønsted-Lowry Theory
- It does not explain the acid-base behavior in aprotic solvents such as benzene and dioxane.
- It fails to explain the reaction between acid oxides (CO2, SO2, and SO3) and basic oxides (BaO, CaO, and Na2O) because there is no proton transfer.
- It fails to recognize the acidic nature of proton-less compounds like AlCl3, FeCl3, and BF3.



Very help ful
very helpful and great concepts wise points