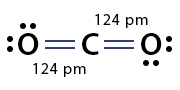Bond Length
What is Bond Length
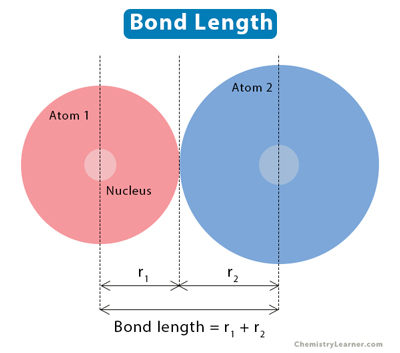
Atoms combine to form chemical bonds. Bond length is the average distance between the nuclei of two bonded atoms in a molecule. The number of electrons bonded to the two atoms determines the bond length [1-4].
Factors Affecting Bond Length [1,6]
1. Bond order (or the number of bonding electrons): The more the number of bonding electrons, the shorter the bond is. Bond length is highest for a single bond and lowest for a triple bond.
single bond > double bond > triple bond
2. Bond strength: The stronger the bond, the shorter will be the bond length. In other words, the bond length decreases with increasing bond strength.
3. Bond disassociation energy: The shorter the bond, the higher the bond strength and, hence, the bond dissociation energy.
How to Find Bond Length
The bond length can also be determined from the Lewis structure of the molecule. Here are the steps.
- Draw the Lewis structure of the compound and identify the bond of interest.
- Look up the covalent radius of the two atoms from the chart.
- Calculate the sum of the two radii.
Example: Carbon dioxide (CO2)
1. Draw the Lewis structure of CO2
2. Find the covalent radii of carbon and oxygen double bonds from the chart: (C=): 67 pm and (O=): 57 pm
3. Add the sum of the two radii: 67 pm + 57 pm = 124 pm
Examples of Bond Lengths
The following table lists and compares the bond lengths between two atoms in all three types of covalent bonds. The values are given in picometer (1 pm = 10-12 m) [7].
| Compound | Bond | Bond length (pm) |
|---|---|---|
| Hydrogen (H2) | H-H | 74 |
| Alkane | C-C | 151 |
| Alkene | C=C | 134 |
| Alkyne | C≡C | 120 |
| Nitrogen (N2) | N-N N=N N≡N | 142 120 108 |
| Oxygen (O2) | O-O O=O O≡O | 129 114 106 |
| Carbon dioxide (CO2) | O=C=O | 116 |
| Water (H2O) | H-O-H | 97 |
| Ammonia (NH3) | N-H | 101 |
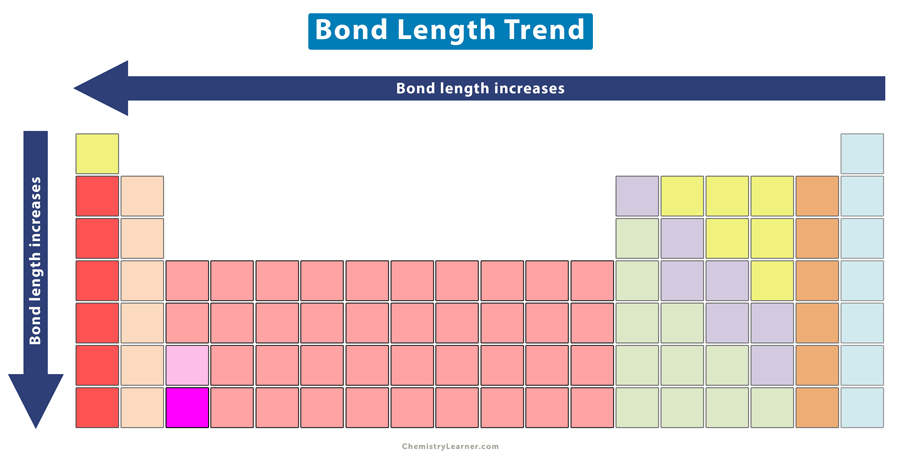
The bond length displays a specific trend in the periodic table.
Bond Length Trend
In order to understand the bond length trend, let us take a look at a few bond lengths of hydrogen and carbon with other atoms.
| Molecule | Bond length (pm) |
| H-H | 74 |
| H-F | 92 |
| H-Cl | 127 |
| H-Br | 141 |
| H-I | 161 |
| C-C | 151 |
| C-N | 147 |
| C-O | 143 |
| C-F | 133 |
| C=C | 134 |
| C=N | 127 |
| C=O | 123 |
The order of bond lengths of carbon and other atoms are as follows:
C-C > C-N > C-O > C-F
C=C > C=N > C=O
It is clear that as the covalent radius of the elements decreases, the bond length also decreases. Hence, the bond length decreases across a period of the periodic table.
The order of bond length of hydrogen halide is as follows:
H-F < H-Cl < H-Br < H-I
It is clear that as the covalent radius of the halogens increases, the bond length also increases. Hence, the bond length increases down a group in the periodic table.
The bond length is related to other properties of the compound. In the following sections, we shall discuss its relationship with other quantities.
Bond Length and Bond Energy
The amount of energy required to break all covalent bonds of the same type in one mole of a compound in a gaseous state is called bond energy. The bond energy is inversely proportional to the bond length. A shorter bond length has higher bond energy. The following table shows the bond length and bond energy of carbon-carbon bonds [8].
| Bond | Bond Length (pm) | Bond Energy (kJ/mol) |
|---|---|---|
| C-C | 151 | 347 |
| C=C | 134 | 614 |
| C≡C | 120 | 839 |
Bond Length and Resonance
Resonance is the delocalization of electrons in a conjugated system. The bond length of a single bond decreases since it gets some double bond character, and that of a double bond increases as it loses some of its character. As a result, the lengths of single and double bonds change to intermediate values. The resonance structures allow one to predict bond length [1].
For this reason, the carbon-carbon bond lengths in benzene are equal. The carbon-carbon bond alternates between C-C and C=C. The bond length between two carbon atoms is 139.9 pm, between C-C (151 pm) and C=C (134 pm).