Aluminum Bromide
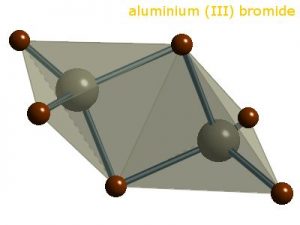
Aluminum bromide, also known by its common name aluminum tribromide and IUPAC name tribromoalumane, is a hygroscopic compound represented by the chemical formula AlBr3 [1, 4]. Its dimeric form (Al2Br6) exists mainly in the solid phase [2].
How is Aluminum Bromide Prepared
It is synthesized by the slow addition of pieces of aluminum foil to liquid bromine, and this direct bromination is represented by the following reaction [5]:
2Al + 3Br2 → 2AlBr3
The equation is highly exothermic and causes the aluminum foil to melt, producing heat and light [5].
Reaction with Chlorine
When aluminum bromide reacts with chlorine, it undergoes single replacement to yield aluminum chloride and bromine, which is shown by the following balanced equation:
2AlBr3+ 3Cl2 → 2AlCl3 + 3Br2
Properties and Characteristics of Aluminum Bromide
General Properties |
|
| Molar Mass/Molecular Weight | 266.69 g/mol [1, 4] |
Physical Properties |
|
| Color and Appearance | White to pale yellowish-red, lumpy powder (anhydrous form) [1, 4] |
| Odor | Pungent [1] |
| Melting Point | 97.5 °C, 207.5 °F (anhydrous) [1] |
| Boiling Point | 255 °C, 491 °F (anhydrous) [1] |
| Density | 3.2 g cm-3 [1, 4] |
| State of matter at room temperature | Solid [1, 4] |
| Solubility | Soluble in benzene, toluene, nitrobenzene, ether, methyl alcohol, and acetone [1] |
| Solubility in Water | Highly soluble, partially hydrolyzes shown by a fuming solution and appearance of white precipitate |
| Heat Capacity (C) | 100.6 J/(mol.K) |
| Lattice Constant (a, b, and c) | 0.7512 nm, 0.7091 nm, and 1.0289 nm |
Atomic Properties |
|
| Crystal Structure | Monoclinic lattice [6] |
What is It Used for
Although it does not have any significant commercial applications, its anhydrous form works as a catalyst for increasing the rate of Friedel-Crafts alkylation reaction [7, 8].
Is Aluminum Bromide Toxic
Exposure through inhalation and swallowing can cause acute toxicity [8]. Contact with skin and eyes can result in irritation, corrosion, and damage [8].
- References
- Aluminum Bromide (Compound) – Pubchem.ncbi.nlm.nih.gov
- Aluminum Bromide – Aluminumsulfate.net
- Aluminium Bromide – Chemspider.com
- Aluminum Bromide – Americanelements.com
- Reaction Between Aluminum and Bromine – Chemed.chem.purdue.edu
- The Crystal Structure of Aluminum Bromide – Onlinelibrary.wiley.com
- Aluminium Bromide (T3D1720) – T3db.ca
- Aluminum Bromide – Chemicalbook.com