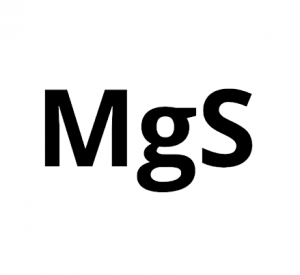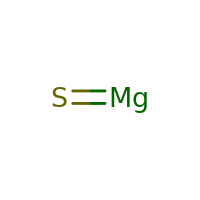Magnesium Sulfide
Magnesium sulfide represented by the chemical formula MgS that bears the IUPAC name sulfanylidenemagnesium [1] is a white crystalline inorganic compound that is moderately soluble in water and acid [3]. It is an ionic compound of magnesium and sulfur [6].
Magnesium Sulfide Identification |
|
| CAS Number | 12032-36-9 [1] |
| PubChem CID | 82824 [1] |
| ChemSpider ID | 8305407 [2] |
| EC Number | 234-771-1 [1] |
How to Make It
Magnesium sulfide can be formed by a reaction between hydrogen sulfide and magnesium [4].
H2S + Mg = MgS + H2
It can also be synthesized by combining sulfur with magnesium [4].
S + Mg = MgS
Properties and Characteristics of Magnesium Sulfide
General Properties |
||
| Molar mass/molecular weight | 56.365 g/mol [1] | |
Physical Properties |
||
| Color/appearance | White to reddish-brown powder [3] | |
| Melting point/freezing point | >2000°C, >3632°F [3] | |
| Boiling point | N/A [3] | |
| Density | 2.68 g cm-3 [3] | |
| State of matter at room temperature (normal phase) | Solid [3] | |
Chemical Properties |
||
| Solubility in water | Decomposes [3] | |
Atomic Properties |
||
| Crystal structure | Halite (cubic) [3] | |
Prominent Reactions
Magnesium sulfide reacts with water forming hydrogen sulfide and magnesium hydroxide [5].
MgS + 2H2O = H2S + Mg(OH)2
It reacts with phosphoric acid to give magnesium phosphate and hydrogen sulfide [7].
3MgS + 2H3PO4 = Mg3(PO4)2 + 3H2S
Magnesium sulfide reacts with hydrochloric acid to produce magnesium chloride and hydrogen sulfide [8].
MgS + 2HCl = MgCl2 + H2S
It reacts with nitric acid giving magnesium nitrate and hydrogen sulfide [9].
MgS + 2HNO3 = Mg(NO3)2 + H2S
Magnesium Sulfide (MgS) Uses
- In the BOS (basic oxygen steelmaking) process for producing steel [4].
- Being a wide band-gap direct semiconductor, it finds application as a blue-green emitter and photodetector for ultraviolet light of short wavelength [4].
- As a lab reagent [10].
Is It Dangerous
It may catch fire if large quantities are self-heated. MgS is acutely toxic when swallowed or brought in contact with skin and eyes leading to eye damage. It is corrosive and very harmful to aquatic life. The compound is hazardous when it comes in contact with moisture producing poisonous hydrogen sulfide gas [1, 4].
- References
- Magnesium Sulfide (MgS) – Pubchem.ncbi.nlm.nih.gov
- Magnesium Sulfide – Chemspider.com
- Magnesium Sulfide – Americanelements.com
- Magnesium Sulfide – Revolvy.com
- What is Magnesium Sulfide – Quicksciencehelp.wordpress.com
- Draw the Lewis dot structure for Mg and S. Is it an ionic or covalent bond? – Enotes.com
- Balanced Chemical Equation – En.intl.chemicalaid.com
- Balanced Chemical Equation – En.intl.chemicalaid.com
- Magnesium sulphite + nitric acid gives? – Answers.yahoo.com
- Magnesium Sulfide – Chemicalbook.com