Electronegativity
What is Electronegativity
Electronegativity is the tendency of an atom to attract a pair of electrons in a chemical bond. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. The more electronegative an atom is, the higher will be the attractive force [1-4].
How to Find Electronegativity Values
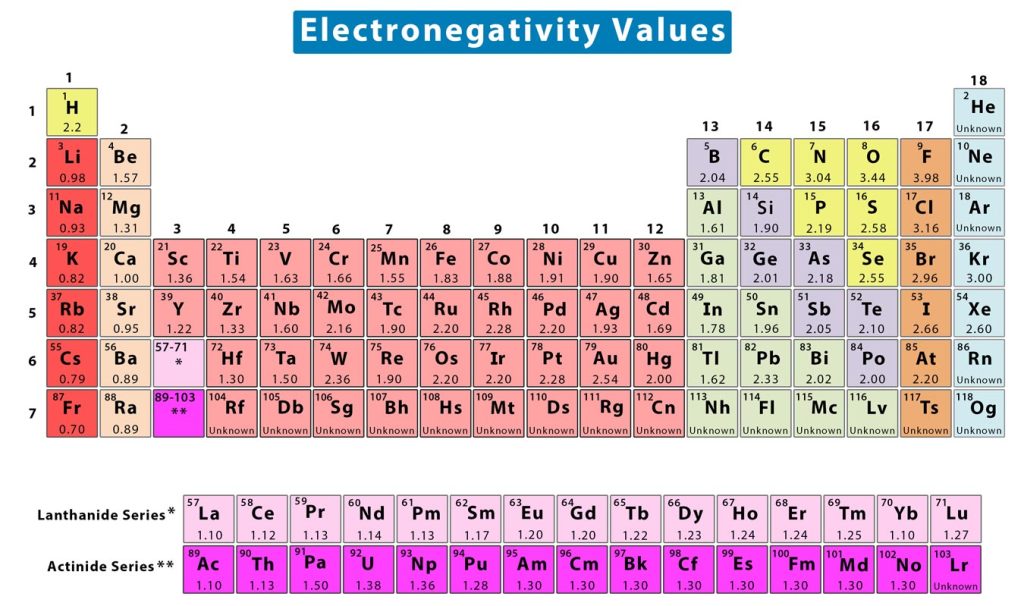
Electronegativity can be quantified using several scales. The most prominent scale is known as the Pauling scale. The scale is named after American chemist Linus Pauling, who is credited with having discovered electronegativity. On this scale, electronegativity is a dimensionless quantity and does not have any unit. This scale can be applied to determine the electronegativity values of the elements in the periodic table. Typically, the values range from 0.7 to 4. The following image shows the periodic table of elements with the electronegativity values [1].
How to Find the Electronegativity Difference
The difference in the electronegativity values of two elements is known as electronegativity difference. This value is an essential quantity as it determines the type of chemical bond formed between two atoms. In order to calculate the electronegativity difference, one can find the electronegativity values from charts and subtract the lower value from the higher one.
How is Electronegativity Related to Bonding
Electronegativity is an important property that affects the bonding between two atoms and hence the molecular properties. The electronegativity difference between two atoms can predict the type of chemical bond that they can form. These bonds are discussed below [1,2,5].
1. Covalent Bond
A covalent bond is formed when the electronegativity difference between the two atoms is equal to or less than 2. The covalent bond can be of two types – polar and nonpolar.
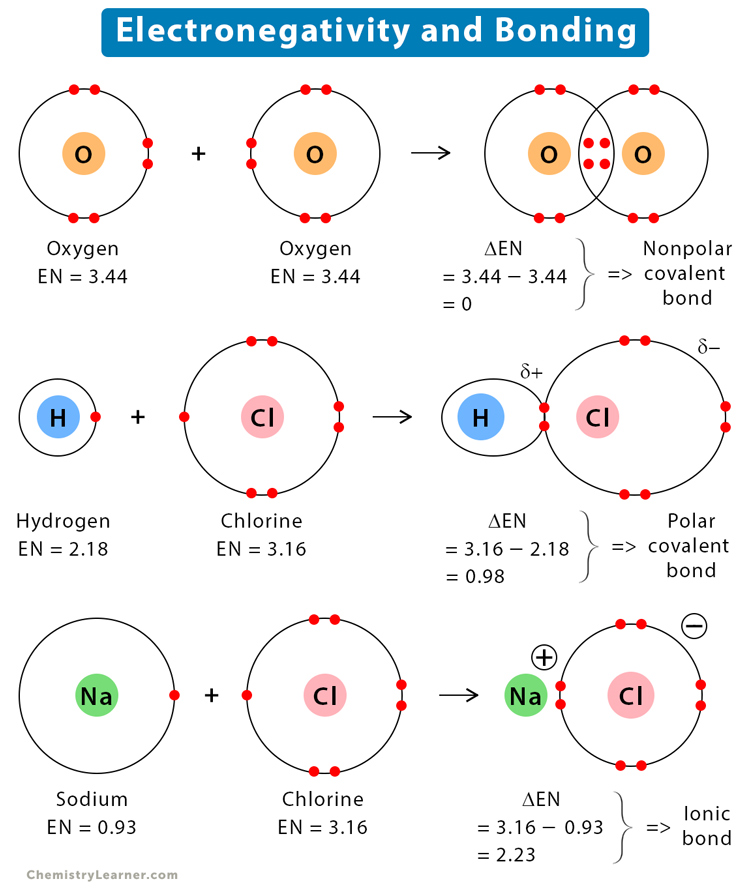
The covalent bond is nonpolar if the electronegativity difference is zero. The following image shows a nonpolar covalent bond between two atoms of A. The electrons are equally shared between the two atoms.
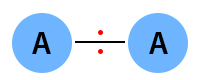
Examples: Hydrogen (H2), oxygen (O2), and chlorine (Cl2)
The covalent bond is polar if the electronegativity difference is between zero and 2. The following image shows the polar covalent bond between A and B. Here, B is more electronegative than A. It influences the bond by pulling the electron pair. The symbol delta (δ) indicates a partial charge.
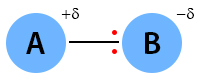
Examples
1. Water (H2O)
The electronegativity of hydrogen (H) is 2.18 and that of oxygen is 3.44. Therefore, the electronegativity difference (ΔEN) is,
3.44 – 2.18 = 1.26
Since the difference is less than 2, it implies that the bond between O and H in H2O is a polar covalent.
2. Hydrogen chloride (HCl)
The electronegativity of hydrogen is 2.18 and that of chlorine (Cl) is 3.16. Therefore, the electronegativity difference is,
3.16 – 2.18 = 0.98
Since the difference is less than 2, it implies that the bond between H and Cl in HCl is polar covalent.
3. Ammonia (NH3)
The electronegativity of hydrogen is 2.18 and that of nitrogen (N) is 3.04. Therefore, the electronegativity difference is,
3.04 – 2.18 = 0.86
Since the difference is less than 2, it implies that the bond between H and N in NH3 is polar covalent.
2. Ionic Bond
An ionic bond exists when the electronegativity difference is greater than 2. The following image shows the ionic bond between A and B.
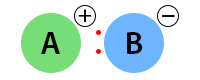
Examples
1. Sodium chloride (NaCl)
The electronegativity of sodium (Na) is 0.93 and that of chlorine is 3.16. Therefore, the electronegativity difference is,
3.16 – 0.93 = 2.23
Since the difference is greater than 2, it implies that the bond between Na and Cl in NaCl is ionic.
2. Magnesium oxide (MgO)
The electronegativity of magnesium (Mg) is 1.31 and that of oxygen is 3.44. Therefore, the electronegativity difference is,
3.44 – 1.31 = 2.13
Since the difference is greater than 2, it implies that the bond between Mg and O in MgO is ionic.
3. Calcium chloride (CaCl2)
The electronegativity of calcium (Ca) is 1 and that of chlorine is 3.16. Therefore, the electronegativity difference is,
3.16 – 1.00 = 2.16
Since the difference is greater than 2, it implies that the bond between Ca and Cl in CaCl2 is ionic.
The following table summarizes the various types of chemical bonds discussed above. It is evident that as the electronegativity difference increases, the bond polarity also increases.
| Type of Chemical Bond | Electronegativity Difference |
|---|---|
| Nonpolar covalent | 0 |
| Slightly polar covalent | 0.1 to 0.4 |
| Polar covalent | 0.5 to 2 |
| Ionic | > 2 |
The following image shows a few examples of nonpolar covalent, polar covalent, and ionic bonds.
In order to explain electronegativity further, let us find out what rules it follows and how it varies among the elements of the periodic table.
Electronegativity Trend in Periodic Table
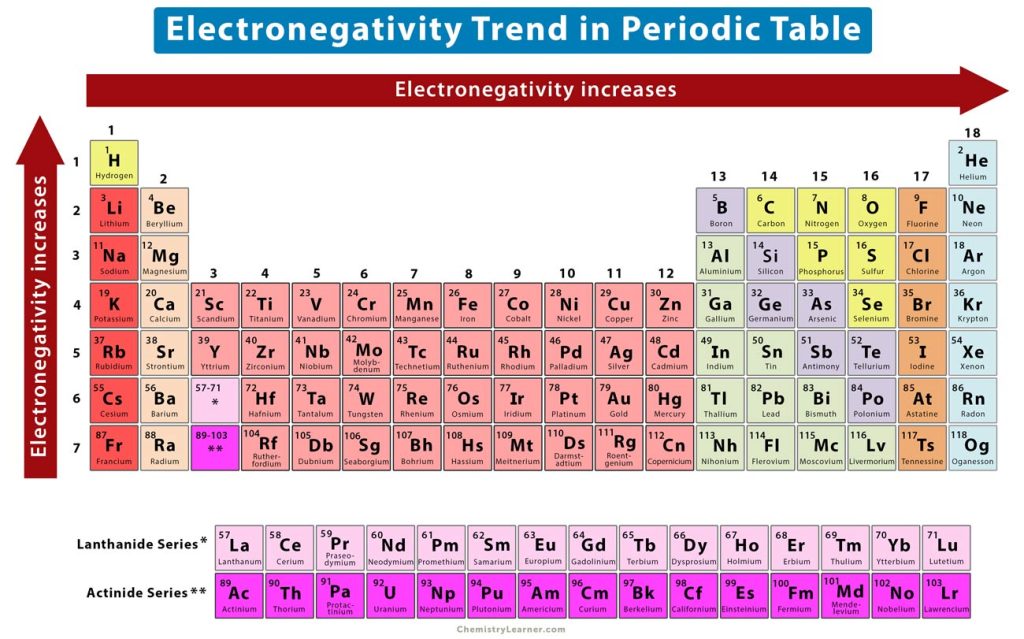
The elements of the periodic table show a specific trend in electronegativity as one moves from left to right across a periodic, and top to bottom down a group [1-4,6].
1. Horizontal Trend: Electronegativity Across a Period
The periodic trend shows that electronegativity increases across a period.
Why does Electronegativity Increase Across a Period
From left to right across a period, the atomic number increases gradually. It means that the number of electrons as well as protons increases. As the number of protons increases, the nuclear charge also increases. As a result, the bonding electrons are pulled towards the nucleus with a higher force and approach closer. Therefore, the electronegativity increases from left to right across a period, as shown in the image above.
2. Vertical Trend: Electronegativity Down a Group
The periodic trend shows that electronegativity decreases down a group.
Why does Electronegativity Decrease Down a Group
The atomic number increases rapidly from top to bottom, thereby increasing the atomic size and atomic radius. As a result, the inner electrons will shield the outer electrons. This shielding effect will decrease the electrostatic attraction between the nucleus and valence electrons, making it difficult for the atom to attract the bonding electrons. Therefore, the electronegativity decreases from top to bottom. In other words, the electronegativity increases from bottom to top, as shown in the image above.
Therefore, the factors affecting electronegativity are atomic number, atomic size and radius, and electron shielding.
Exceptions
Some exceptions to the above trend include noble gases, lanthanides, and actinides. The noble gases lie on the right of the periodic table, have a complete valence shell. Most of them do not attract any electrons and their electronegativities are unknown, except krypton and xenon. On the other hand, the lanthanides and actinides have complex atomic structures and do not follow any electronegativity trend.
FAQs
Ans. Fluorine is the most electronegative element with an electronegativity value of 3.98. The reason is that it has a small atomic radius which makes it easy to attract electrons.
Ans. Francium is the least electronegative element with an electronegativity value of 0.7.
Ans. Chemically reactive metals form bonds by donating electrons and do not tend to attract electrons. These atoms lie on the lower left of the periodic table and hence have low electronegativity values.
Ans. The more electronegative an element is, the better is its ability to retain the electron. Hence, the stability of the atom increases. In other words, an increase in electronegativity increases the atomic strength.