Post-Transition Metals
Definition : What are Post-Transition Metals
The post-transition elements in the periodic table are a group of elements located between the transition metals (to the right) and metalloids (to the left). Due to their properties, they are also called ‘other’ or ‘poor’ metals [1].
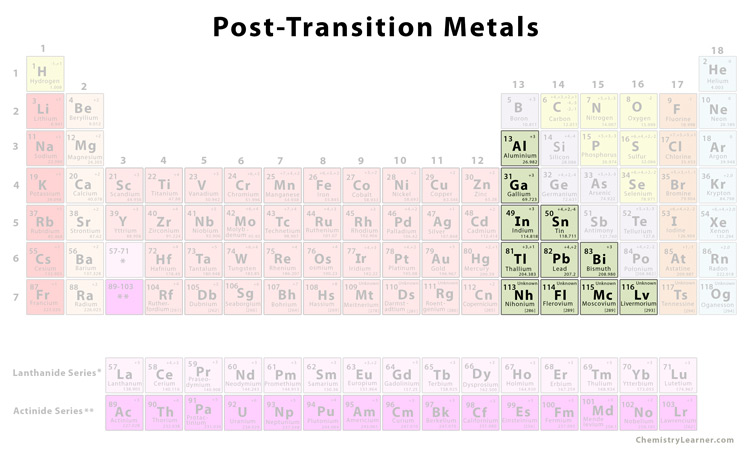
Location of the Post-Transition Metals in the Periodic Table
Although there are some conflicting theories, generally the post-transition metals include elements from groups 13-15 [1,2].
List of Post-Transition Metals |
|
| Aluminum | Al |
| Gallium | Ga |
| Indium | In |
| Tin | Sn |
| Thallium | Tl |
| Lead | Pb |
| Bismuth | Bi |
The elements 113-116 on the periodic table, i.e. nihonium (Nh), flerovium (Fl), moscovium (Mc), and livermorium (Lv), are considered to possibly belong to the post-transition metal family, though it is yet to be confirmed due to some unknown properties of the elements [6].
Polonium is sometimes also included in the list of post-transition metals. The same may be done for zinc, cadmium, and mercury (otherwise considered transition metals) and for germanium and antimony (otherwise considered metalloids) [1].
Properties and Characteristics of Post-Transition Metals
Physical Properties
- Soft or brittle, poor mechanical strength [3]
- Melting points lower than transition metals
- Boiling points are also usually lower than transition metals [3]
- Covalent or directional bonding is shown by crystalline structures [4]
- High density [5]
Chemical Properties
- Tendency to form covalent bonds [3]
- Acid-Base amphoterism
- Can form half-metallic compounds [5]
Periodic Trends of Post-Transition Metals
Generally, atomic radii decrease, and ionization energies increase. As a result, fewer electrons are available for metallic bonding, and so ions are smaller, more polarising, and tend to form covalent bonds. Hence they show lesser metallic nature [3].
Uses of Post-Transition Metals
Different elements in this family have different uses. Aluminium and Tin are respectively used for making utensils in electronics, as well as for soldering and plating steel [5]. Bismuth is used to make Pepto-Bismol, a drug used to soothe upset stomachs [1,5]. Indium is used for electronics, for example, making touch screens and flat panel displays, while Gallium has applications in semiconductors and fuel cells [1, 2]. Lead is used in making batteries, among other things.
Interesting Facts
- Aluminum is the most abundant post-transition metal and the third most abundant element on Earth [1].
- The post-transition metal bismuth was considered to be the heaviest stable element until recently, before it was discovered to be mildly radioactive.
References