Polarity of Sulfur Dioxide (SO2)
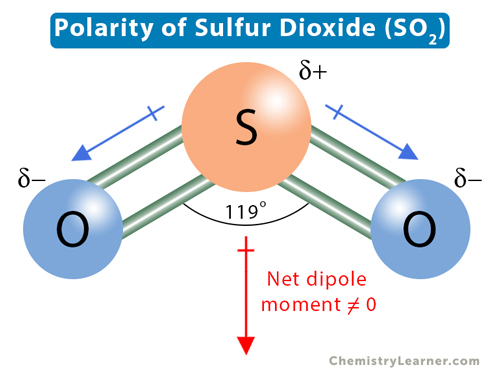
Sulfur dioxide (SO2) consists of one atom of sulfur (S) and two atoms of oxygen (O). Sulfur shares a double bond with each oxygen atom. A lone electron pair on sulfur repels oxygen’s lone pairs, making SO2 a bent structure with a bond angle of 119°.
Oxygen is more electronegative than sulfur. The electronegativity makes the oxygen-sulfur bond polar. The shared electron pairs are attracted toward oxygen, resulting in a partial negative charge on the oxygen and a partial positive charge on the sulfur. Besides, the bent structure of SO2 makes the charge distribution asymmetric, and two poles of charges are observed on the molecule. Such electron distribution makes the SO2 molecule polar.