Polarity of Hydrogen Fluoride (HF)
Hydrogen (H) has one valence electron, and fluorine (F) has seven valence electrons. Both require one electron to complete their outermost shell. We need to know the electronegativity difference between the two atoms to understand what kind of bonds are formed between H and F.
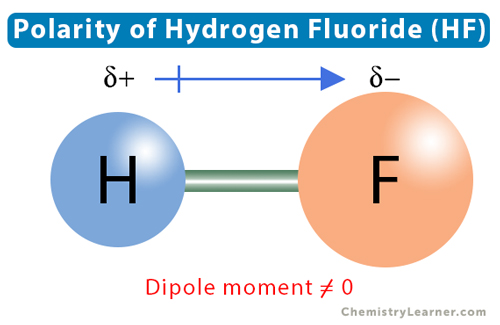
The electronegativity of hydrogen is 2.2, and that of fluorine is 3.98. The difference between the two is 3.98 – 2.2 = 1.78. Therefore, the H-F bond is a polar covalent. Hydrogen and fluorine will each share one electron. Hydrogen will acquire a partial positive charge, and fluorine will acquire a partial negative charge. The dipole moment vector will direct from H to F.