Polarity of Carbon Tetrafluoride (CF4)
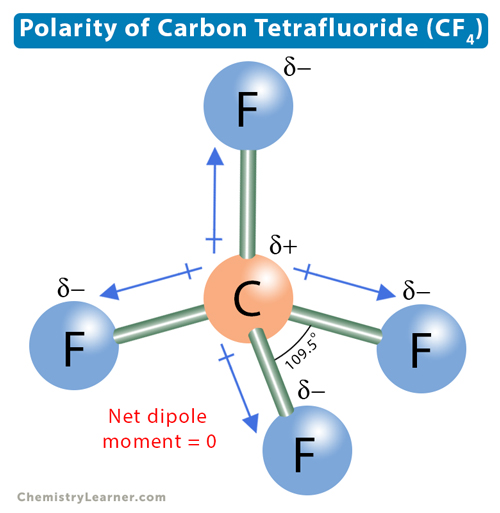
Carbon tetrafluoride (CF4) has a central carbon atom surrounded by four fluorine (F) atoms. Carbon has four valence electrons, and fluorine has seven. Hence, carbon requires four more electrons, and fluorine requires one electron to complete its octet. Therefore, carbon bonds with four fluorine atoms through single covalent bonds, resulting in a tetragonal structure. In such a structure, all bonds are equidistant with a bond angle of 109.5°.
The electronegativity of carbon is 2.55, and that of fluorine is 3.98. Carbon is the least electronegative atom and sits at the molecule’s center. Fluorine is the most electronegative atom and attracts the shared electron pair toward itself. The electronegative difference between the two atoms and the ability of fluorine to attract bonding electron pairs make the C-F bond polar. There will be a dipole moment directed from C to F. However, the dipole moments cancel due to tetragonal symmetry, making CF4 nonpolar.