Polarity of Carbon Tetrachloride (CCl4)
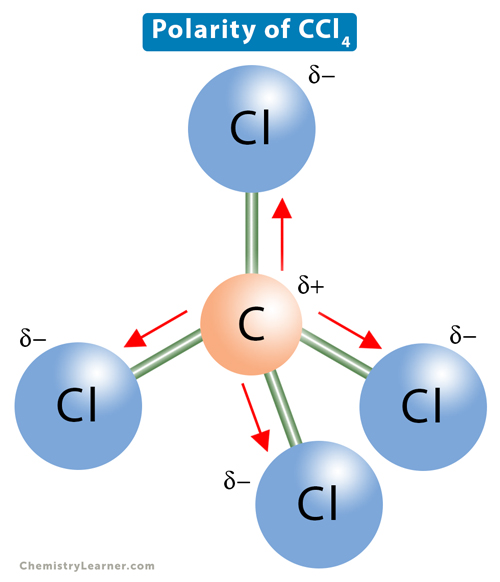
Carbon tetrachloride (CCl4) consists of a central carbon (C) atom with a coordination number 4. It is bonded to four chlorine (Cl) atoms through single covalent bonds. The bonds coordinate around carbon so that the distance between them is maximum while the repulsion between the pairs of bonding electrons is minimum. Such arrangement leads to a tetrahedral geometry with a bond angle of 109.5° [1-4].
The electronegativity of carbon is 2.55, and that of chlorine is 3.16. The electronegativity difference is 0.61. Therefore, the C-Cl bond is polar, with the carbon atom acquiring a partial positive charge and the chlorine atom acquiring a partial negative charge. The bond polarity itself cannot determine the molecular polarity. The net dipole moment of CCl4 is the vector sum of all the dipole moments due to C-Cl bonds. Because of the tetrahedral symmetry, the individual dipole moments will cancel, and the net dipole moment of the molecule is a zero. Therefore, the symmetric charge distribution of carbon tetrachloride makes it nonpolar.