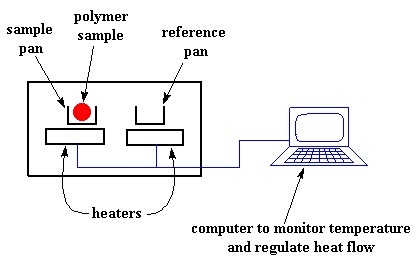
Beryllium copper
Beryllium copper is an alloy comprising of copper as the base and 0.5 to 3 % of beryllium. In some cases, other alloying elements like nickel or cobalt are also used. The alloy has a huge demand in innumerable industries due to its high strength in adjunct with non-magnetic and non-sparking properties. Beryllium...