Zero-order Reaction
A chemical reaction in which the rate is independent of the reactants’ concentrations is called a zero-order reaction. The reaction rate does not change as the reaction proceeds with time [1-4].
A zero-order reaction is due to any of the following two conditions:
- A small portion of the reactant from a large “pool” is available for the reaction. Once that portion is used, it is immediately refilled from the pool.
- One of the reactants is present in a vast concentration or is a heterogeneous catalyst.
Formula
Since the rate is independent of concentration, it can be written as proportional to the zeroth power of the concentration [1-4].
Rate = k [A]0
=> R = k
The unit for the reaction rate is moles per liter per second or mol L-1 s-1. Hence, the unit for the reaction coefficient is also mol L-1 s-1.
Differential Form
The reaction rate is the decrease in reactants’ concentration per time. Therefore, the differential form of the zero-order rate equation is given by
-d[A]/dt = k
Integrated Form
Rearranging the above equation and integrating it on both sides gives the integrated rate equation.
∫d[A] = -k ∫dt
=> [A] = [A]o – kt
Where [A]o is the concentration at t = 0 and [A] is the concentration at time t.
Graphs
We have seen that the rate remains constant in a zero-order reaction, and the concentration is linear with time. Therefore, the plot of [A] vs. t will yield a straight line, as shown below [1-4].
Half-life
The half-life of a reaction is the time frame in which a reactant’s concentration is reduced to one-half of its initial concentration. It can be calculated as follows [1-4]:
At half-life, t = t1/2 and [A] = [A]o/2. Plugging these in the equation for concentration, we get
[A]o/2 = [A]o – kt1/2
=> t1/2 = [A]o/2k
Therefore, the half-life of a zero-order reaction depends on the initial concentration and the rate constant.
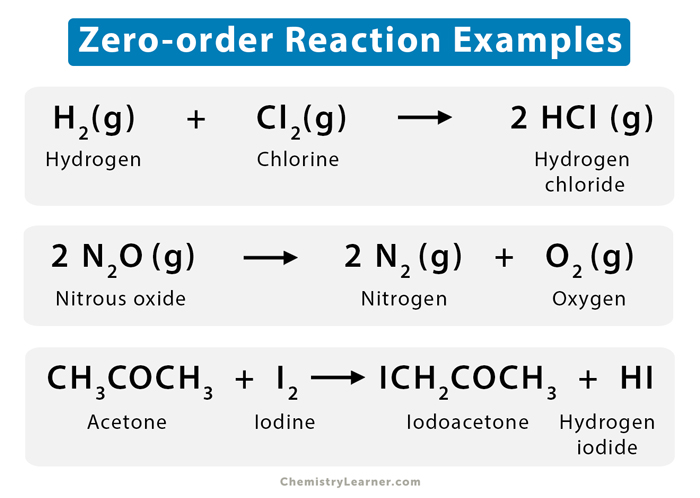
Examples
Reactions catalyzed by enzymes and solid surfaces (heterogeneous catalysis) are typically zero-order reactions. Let us look at a few examples [1-4].
- Hydrogen (H2) reacts with chlorine (Cl2) in the presence of ultraviolet light to give hydrogen chloride (HCl).
H2 (g) + Cl2 (g) → 2 HCl (g)
Such a type of reaction is known as a photochemical reaction.
- Nitrous oxide (N2O) decomposes over a hot platinum surface that is at a temperature of 200 to 400 ˚C to give nitrogen (N2) and oxygen (O2).
2 N2O (g) → 2 N2 (g) + O2 (g)
- Decomposition of ammonia (NH3) in the presence of a molybdenum (Mo) catalyst to give nitrogen (N2) and hydrogen (H2).
2 NH3 (g) → N2 (g) + 3 H2 (g)
This reaction is the inverse of Haber’s process.
Besides, enzyme-catalyzed reactions common in the human body are zero-order reactions. For example, yeast contains enzymes. One of them, zymase, converts glucose into alcohol.