Molecular Geometry of Hydrogen Sulfide (H2S)
Hydrogen sulfide (H2S) molecule consists of one sulfur (S) atom and two hydrogen (H) atoms. Hydrogen (H) is located in Group 1, and sulfur (S) is in Group 16 of the periodic table. Hydrogen has one, and sulfur has six valence electrons. The total number of valence electrons in hydrogen sulfide is 8.
Lewis dot structure represents the valence electrons participating in the bond formation and the nonbonding electrons remaining as lone pairs on the atoms. Dashes in Lewis structure represent bonds, and dots represent lone pairs. The structure is constructed using the octet rule [1-4].
Hydrogen requires one electron to fill its valence shell and attain the electron configuration of its nearest neighbor, neon. Sulfur requires two electrons to complete its octet. By placing sulfur in the middle and hydrogen at the two ends, we can show the bond formation in hydrogen sulfide. The two hydrogens will form single covalent bonds with sulfur. Thus, two out of six electrons in sulfur participate in bond formation, leaving two lone pairs. Therefore, the Lewis structure of H2S shows two sigma bonds and two lone pairs, leading to sp3 hybridization and a steric number of 4.
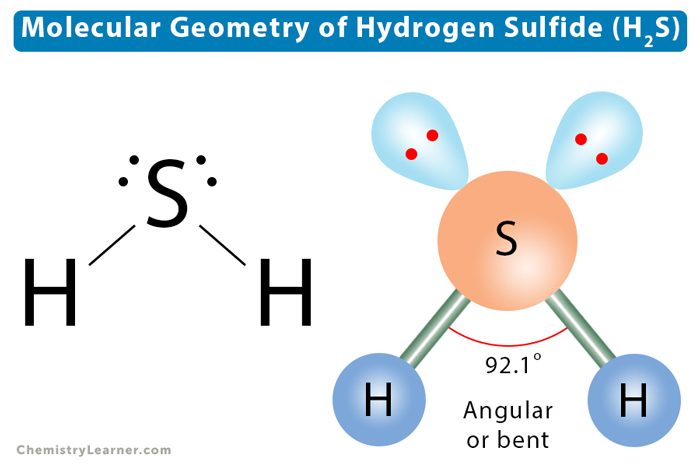
The VSEPR theory is an accurate method to predict molecular geometry. According to this theory, the electron geometry of hydrogen sulfide is tetragonal. The two lone pairs occupy positions as far apart as possible to minimize their repulsion. The electronic repulsion decreases according to the following order.
lone pair – lone pair > lone pair-bond pair > bond pair-bond pair
As a result, the S-H bonds will bend toward each other and away from the lone pairs. The shape of the molecule will be distorted from the ideal tetrahedral shape. Hydrogen sulfide will acquire an angular or bent shape with a bond angle of 92.1°. This angle is less than the ideal tetrahedral bond angle of 109.5°. The VSEPR notation is AX2E2.