Molecular Geometry of Carbon Dioxide (CO2)
The molecular formula of carbon dioxide (CO2) indicates that it has one carbon (C) atom and two oxygen (O) atoms. Carbon lies in Group 14, and oxygen in Group 16 of the periodic table. Carbon and oxygen have four and six valence electrons, respectively. Carbon requires four electrons, and oxygen needs two to complete its valence shells [1-4].
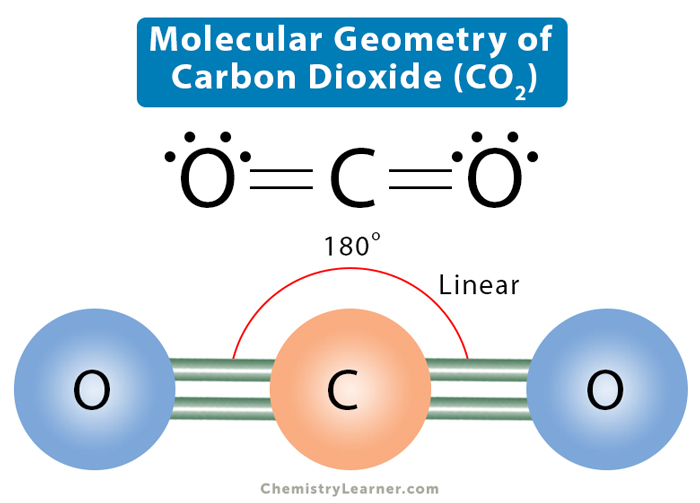
Lewis structure represents how bonds are formed in molecules. Lines indicate bonds and dots depict lone pairs. Carbon is the least electronegative among the two atoms and occupies the central position. The two oxygen atoms occupy the terminal positions and will form bonds with the central carbon atom. The total number of valence electrons in the carbon dioxide molecule is 16. These 16 electrons are distributed so that oxygen and carbon have complete valence shells, fulfilling the octet rule. The only way possible is if the bond between carbon and oxygen is double, leaving no lone pairs on carbon. Each oxygen atom will have two lone pairs.
From the Lewis dot structure, the CO2 molecule will have two regions of electron density around the central carbon atom. VSEPR theory is an accurate way of predicting the shape of a molecule. According to this theory, the bond pairs will stay as far apart as possible so that the repulsion is minimum. Such an arrangement is only possible when the O-C-O bond angle of 180°. Therefore, CO2 is a linear molecule. The VSEPR notation is AX2.