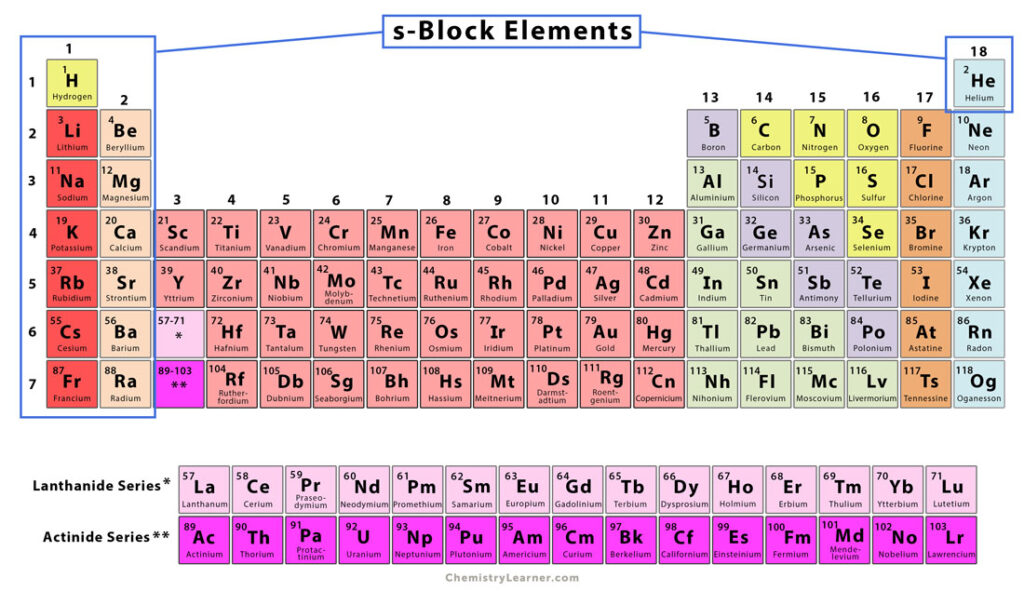
s-Block Elements
The elements of Groups 1 and 2 of the periodic table are called s-block elements. They include alkali and alkaline earth metals. They are called s-block because their valence electrons lie in the s orbital [1-4].
Electron Configuration
Group 1 Elements
The Group 1 elements or alkali metals have one electron in their valence shell. Their electron configuration ends in ns1, where n is the period number. A list of Group 1 elements, their chemical symbols, and electron configurations are shown below [1-4].
| Element Name | Chemical Symbol | Electron configuration |
|---|---|---|
| Hydrogen | H | 1s1 |
| Lithium | Li | [He] 2s1 |
| Sodium | Na | [Ne] 3s1 |
| Potassium | K | [Ar] 4s1 |
| Rubidium | Rb | [Kr] 5s1 |
| Cesium | Cs | [Xe] 6s1 |
| Francium | Fr | [Rn] 7s1 |
Group 2 Elements
The Group 2 elements or alkaline earth metals have two electrons in their outermost shell with an electron configuration ending in ns2. A list of Group 2 elements, with their chemical symbols and electron configurations, are shown below [1-4].
| Element Name | Chemical Symbol | Electron configuration |
|---|---|---|
| Beryllium | Be | [He] 2s1 |
| Magnesium | Mg | [Ne] 3s2 |
| Calcium | Ca | [Ar] 4s2 |
| Strontium | Sr | [Kr] 5s2 |
| Barium | Ba | [Xe] 6s2 |
| Radium | Ra | [Rn] 7s2 |
Note: Helium is considered an s-block element since its valence electrons lie in the 1s subshell. However, it is located in Group 18. Its electron configuration is 1s2.
Properties [1-4]
Physical Properties
1. All the s-block elements are light metals except hydrogen, which is a non-metal. They are soft, silvery, shiny, and good conductors of electricity and heat.
2. The alkali metals have low melting and boiling points due to weak metallic bonding. They have low density, increasing Group 1 from Li to Cs. The exception is K, which is lighter than Na. Li is the lightest known metal. Fr is a natural element that can be found rarely on Earth.
3. The alkaline earth metals are harder than alkali metals. Due to their smaller sizes, their melting and boiling points are higher than the corresponding alkali metals.
Chemical Properties
1. The outermost electrons are loosely held to the atom. As a result, these metals quickly lose their valence electrons and are highly electropositive.
2. Alkali metals lose one valence electron and form a +1 oxidation state. Alkaline earth metals lose two valence electrons and form a +2 oxidation state.
3. Alkali and alkaline earth metals react vigorously with water to produce hydrogen gas. They must be stored carefully to prevent unnecessary reactions or even explosions.
4. Alkali metals react with hydrogen to produce hydrides. These hydrides react with water to form the corresponding hydroxides and hydrogen gas.
Example
2 Li + H2 → 2 LiH
LiH + H2O → LiOH + H2
Alkali metals also form complex hydrides such as LiAlH4.
5. Alkali metals react with oxygen to produce oxides and superoxide. Examples: Li2O and Na2O2.
6. Fireworks are composed of s-block elements along with other substances. Their purple, red, and green colors are due to the ionic forms of potassium, strontium, and barium.
Diagonal Relationship
A diagonal relationship exists between the elements of the second and third periods. For instance, lithium in Group 1, Period 2 shows similar properties to magnesium in Group 2, period 3. Similarly, beryllium in Group 2, Period 2 shows similar properties to aluminum in Group 13, Period 3. Two elements showing identical properties are called diagonal pairs or diagonal neighbors [1-4].
Many similarities exist between lithium and magnesium, and one of them is the atomic size. The following table shows the atomic and ionic radii of the two elements.
| Property | Lithium | Magnesium |
|---|---|---|
| Atomic radius | 152 pm | 160 pm |
| Ionic radius | 76 pm | 72 pm |
Many similarities also exist between beryllium and aluminum. The ionic radius of Be2+ is 31 pm, and that of Al3+ is 50 pm. Both elements have nearly the same charge/radius ratio.