First Law of Thermodynamics
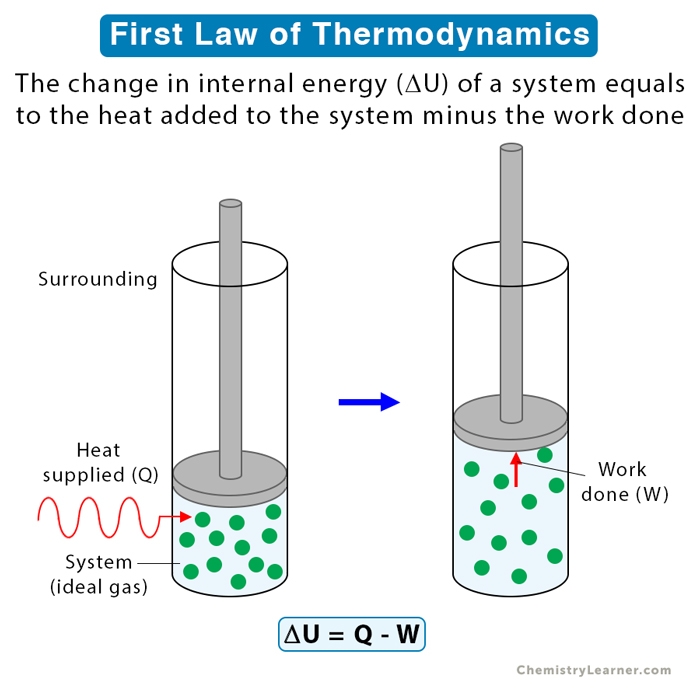
The first law of thermodynamics uses the principles of energy conservation. It establishes a relationship between the internal energy of a system, heat transfer, and work done. According to the first law, the change in internal energy equals the heat supplied to the system minus the work done by the system [1-4].
The conservation of energy principle states that energy can neither created nor destroyed. It transfers from one form to another.
German physicist Rudolf Clausius was the first person to lay down the statement of the first law in 1850.
Equation
Consider a system and its surroundings. Mathematically, the first law of thermodynamics is given by the following equation [1-6].
ΔU = Q – W
Where,
ΔU: Change in internal energy of the system
Q: Heat added to the system
W: Work done by the system
Unit: Joule or J
Dimension: [ML2T-2]
The first law assumes that no matter transfers between the system and the surroundings. Only energy in the form of heat is exchanged. This type of system is known as a closed system.
When work is done on the system, the first law equation modifies to
ΔU = Q + W
The equations are equivalent since
Work done by the system = – Work done on the system
When work is done on the system, it increases internal energy. Hence, the positive sign in ΔU = Q + W equation. When the system does work, its internal energy is reduced. Hence, the negative sign in ΔU = Q – W equation.
For an isolated system, where there is no heat and matter exchange between the system and the surroundings, Q = 0 and ΔU = – W.
Examples [6]
- The melting of ice cubes is a typical example of the first law of thermodynamics. Ice absorbs heat from the surroundings and cools the air. The heat goes on to melt the ice.
- Eating is an example of the first law. Human metabolism involves converting food into heat, work, and stored fat.
- Turning on the electric kettle is another example of the first law. When we turn the switch on, the electricity fires the heater, which warms the water.
First Law and Heat Engines
The most common application of the first law is heat engines. Heat engines convert thermal energy into mechanical energy and vice versa. The basic principle of heat engines is that they establish a relationship between heat, volume, and pressure of a working fluid, like gas [1,4].
When gas is heated, it expands. However, if it is prevented from expanding, it applies pressure to the walls of the container. Suppose the gas is confined inside a cylinder with a movable piston. In that case, the gas applies force on the piston’s surface, allowing it to move. The piston’s movement can be harnessed to do work, whose value is given by the product of the force and the distance the piston moves.
The first law necessitates that heat engines cannot produce more work than the heat supplied. This limitation implies that the engine’s thermal efficiency, defined as the ratio of the work done to the heat supplied, cannot be greater than 100%.
First Law and Perpetual Motion
A perpetual motion machine is a hypothetical device that produces work without an energy supply. It violates the first law. Such a type of machine is impossible to build. Since it violates the first law, it is often called a perpetual machine of the first kind [1].
References
- What is the First Law of Thermodynamics? – Livescience.com
- First Law of Thermodynamics – Grc.nasa.gov
- First Law of Thermodynamics – Hyperphysics.phy-astr.gsu.edu
- The first law of thermodynamics: What is it? – Space.com
- What is the First Law of Thermodynamics? – Khanacademy.org
- The First Law of Thermodynamics – Phys.libretexts.org