Laws of Thermodynamics
The laws of thermodynamics are a set of laws that characterizes a physical system in thermal equilibrium. They define several valuable thermodynamic quantities like internal energy, heat, temperature, enthalpy, and entropy and establish relationships among these parameters. These laws are the fundamental laws of physics and are used in other branches of natural science [1-4].
There are four laws of thermodynamics – zeroth law, first law, second law, and third law.
What are the Laws of Thermodynamics [1-5]
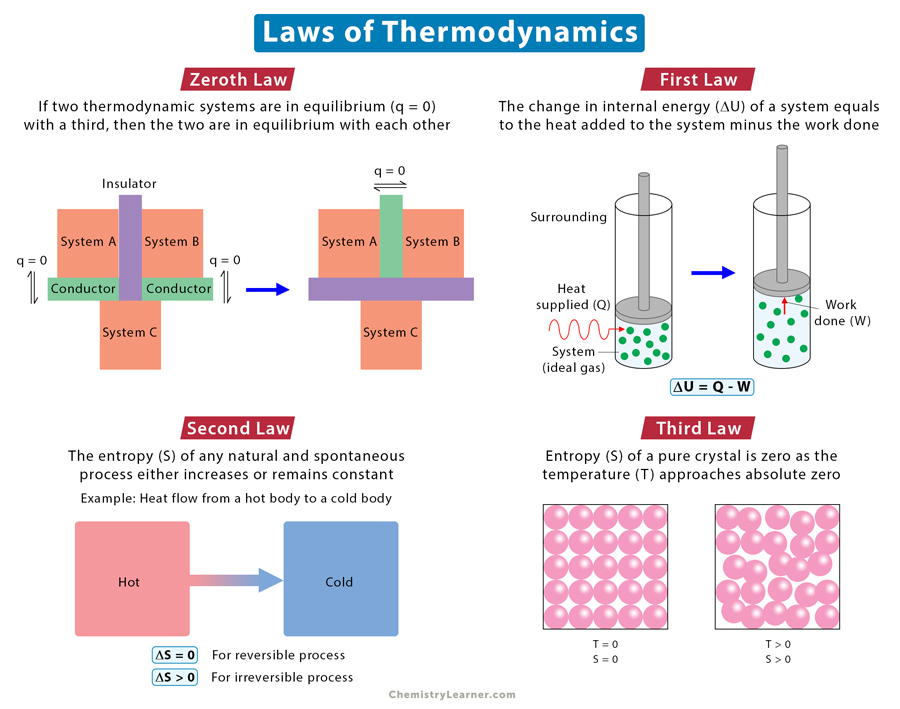
Zeroth Law
Statement: If two thermodynamic systems are in thermal equilibrium with a third one, then the two are in thermal equilibrium with each other.
The zeroth law defines thermal equilibrium and forms the basis for defining temperature. A consequence of the zeroth law is that measuring temperature started to gain importance. When a thermometer measures an object’s temperature, the mercury is in equilibrium with the object. If the same thermometer measures another object’s temperature, it will come to equilibrium with that object.
First Law
Statement: When energy is supplied to or removed from a system, the system’s internal energy changes according to the conservation energy principles.
In other words, energy can never be created or destroyed. It transfers from one form to another. A gain in energy by the system equals the loss in energy by the surroundings. Similarly, a loss in energy by the system equals to gain in energy by the surroundings.
Second Law
Statement: The combined entropy of a system and its surroundings in a natural and spontaneous process never decreases.
An alternative way of stating the second law is that every energy transfer that takes place increases the total entropy of the universe or remains constant (for a reversible process). In other words, the transfer process reduces the valuable energy needed to do work. The second law determines the direction in which the process will occur. A process, such as a chemical reaction, proceeds in a direction that will increase the universe’s overall entropy.
Third Law
Statement: The entropy of a perfect crystal of a pure substance is zero as the temperature approaches absolute zero or 0 K.
A perfect crystal does not have any defects. All atoms, ions, or molecules are in well-defined positions in a structured lattice. The third law implies that a perfect crystal at absolute zero must exist in a single microstate. The significance of the third law is that it allows us to measure the absolute entropy of any pure substance at any temperature, using zero Kelvin (0 K) as the reference temperature.