Adsorption Chromatography
Adsorption chromatography is a technique used in analytical chemistry to separate and analyze components of a mixture based on their affinity for an adsorbent material. In this process, the sample mixture is passed through a column containing the adsorbent, and different components interact with the adsorbent to varying degrees.
Principles of Adsorption Chromatography
Adsorption chromatography is based on the principle of interactions between the stationary phase (adsorbent) and the mobile phase (sample). The working principle of adsorption chromatography involves separating components in a mixture based on their differential affinities for the stationary phase.
The principles of adsorbents used in chromatography play a crucial role in this process. The adsorbent material selectively interacts with different components of the sample, causing them to move at different rates through the column. The more strongly a component interacts with the adsorbent, the slower it progresses through the column, resulting in separation from other components. In essence, adsorption chromatography relies on the selective retention of analytes by an adsorbent material to achieve separation and analysis.
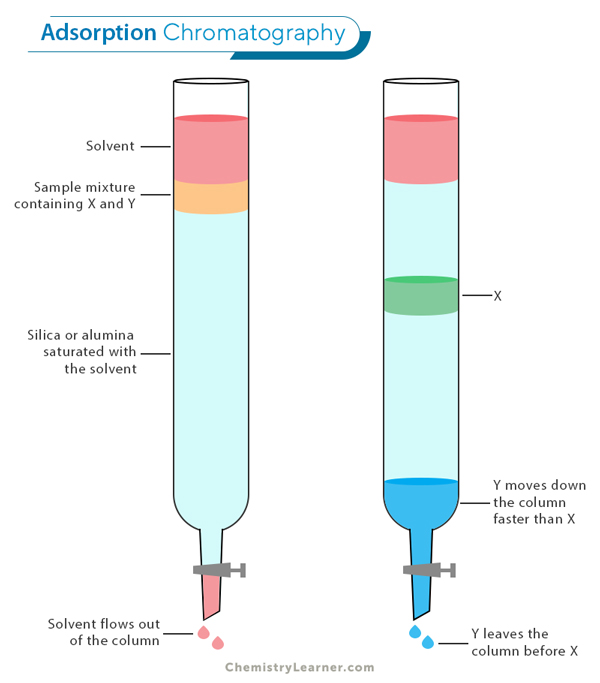
Procedure of Adsorption Chromatography
The first step of adsorption chromatography is to prepare the adsorption column by packing it with the appropriate stationary phase material. The sample mixture containing the compounds of interest is then applied to the top of the column.
Next, a solvent or mobile phase is passed through the column, allowing the different compounds in the sample to interact with the stationary phase. As each compound interacts with the stationary phase to varying degrees, they are separated based on their affinity and elute from the column at different times.
During elution, eluent fractions containing separated compounds are collected regularly. These fractions can then be further analyzed or processed as needed.
Applications of Adsorption Chromatography
Due to its versatility and effectiveness, adsorption chromatography plays a crucial role in research in various industries.
- Separation of Mixtures: Adsorption chromatography is widely used to separate complex mixtures into individual components. The different affinities of substances for the adsorbent result in varied retention times, allowing for effective separation.
- Purification of Compounds: This technique is employed to purify target compounds from impurities. By selecting appropriate adsorbents and optimizing conditions, impurities can be selectively adsorbed, leaving the purified compound behind.
- Analytical Chemistry: Adsorption chromatography is commonly used in analytical laboratories for qualitative and quantitative analysis of various substances. It provides a valuable tool for identifying and quantifying components in a sample.
- Quality Control in Industries: Industries such as pharmaceuticals, food, and chemicals use adsorption chromatography for quality control. It helps ensure the consistency and purity of products by verifying the presence of specific components and detecting impurities.
- Environmental Monitoring: Adsorption chromatography plays a crucial role in environmental analysis. It assesses the presence of pollutants, toxins, and contaminants in air, water, and soil samples, aiding in environmental monitoring and compliance with regulations.
- Biomedical Applications: In biomedicine, adsorption chromatography isolates and purifies biomolecules, such as proteins and nucleic acids. This is vital in various research, diagnostic, and therapeutic applications.
- Drug Development: Adsorption chromatography is applied in the pharmaceutical industry during drug development. It helps in the isolation and characterization of active pharmaceutical ingredients (APIs) and the analysis of drug formulations.
- Study of Surface Interactions: Adsorption chromatography provides insights into the surface interactions between molecules and the adsorbent material. This information is valuable for understanding the behavior of different substances in the chromatographic process.
- Chiral Separations: The technique is utilized for chiral separations, where enantiomers (mirror-image isomers) are separated based on their different affinities to the chiral stationary phase, aiding in producing enantiomerically pure compounds.
Types of Adsorption Chromatography
Adsorption chromatography encompasses various methods, each tailored to specific applications. Among the diverse types, column chromatography, thin-layer chromatography (TLC), gas-solid chromatography, and paper chromatography stand out as prominent approaches.
- Column chromatography separates components based on differential adsorption onto a stationary phase within a vertical column. The stationary phase, often silica or alumina, interacts with the sample molecules as the mobile phase elutes through the column. This process allows for separating compounds based on their affinity for the stationary phase, with different components traveling at varying rates and eventually being collected at distinct points.
- Thin-layer chromatography is a planar chromatographic technique where a thin layer of stationary phase, typically coated on a glass or aluminum support, serves as the separation medium. A small quantity of the sample is applied near the base of the thin layer, and the components migrate up the plate as the mobile phase ascends. The distinct positions of the components on the plate visualize the separation.
- Gas-solid chromatography relies on the adsorption of gaseous analytes onto a solid stationary phase. In this method, a gaseous mobile phase transports the sample through a column containing the stationary phase, leading to differential adsorption of components based on their interaction with the solid surface. Gas-solid chromatography is widely employed to analyze volatile compounds, offering high sensitivity and resolution.
- Paper chromatography utilizes a sheet of absorbent paper as the stationary phase and a liquid mobile phase to separate components. The sample is applied to the paper near one end, and as the solvent moves through the paper, the components are carried along at different rates. This technique is simple, cost-effective, and often employed in educational and qualitative analyses.